Glimepiride tablet and preparation method thereof
A technology of glimepiride tablet and urea tablet, which is applied in the field of medicine, can solve the problems of lowering the qualified rate of glimepiride, poor repeatability of treatment effect, poor disintegration rate and dissolution rate, etc., so as to improve bioavailability , Reduce the effect of strong hygroscopicity and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
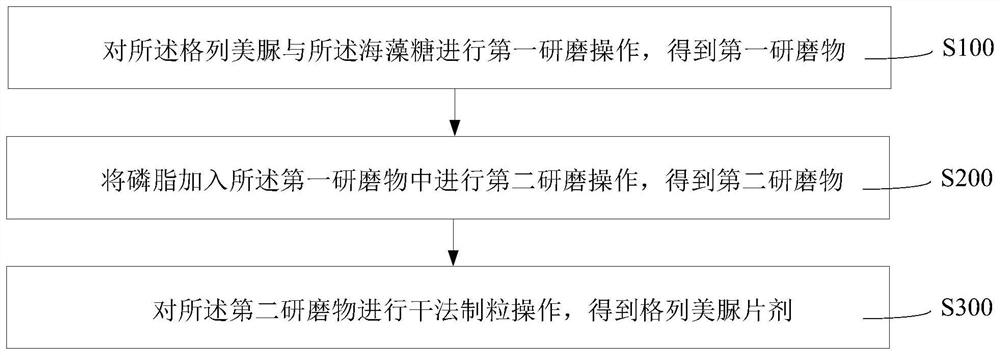
[0073] The present application also provides a preparation method of a glimepiride tablet, which is used for preparing the glimepiride tablet described in any of the above-mentioned embodiments. The preparation method of the glimepiride tablet comprises the following steps: performing a first grinding operation on glimepiride and trehalose to obtain a first grinding product; adding phospholipids to the first grinding material to perform a second grinding operation to obtain a second grinding product Grinding product; dry granulation operation is performed on the second grinding product to obtain glimepiride tablet.
[0074] The preparation method of the above-mentioned glimepiride tablet makes glimepiride and trehalose carry out the first grinding operation, which effectively weakens the hygroscopicity in the pulverization process of glimepiride, and in the grinding process, trehalose and Glimepiride forms a mixed system with better uniformity. In addition, under the condition...
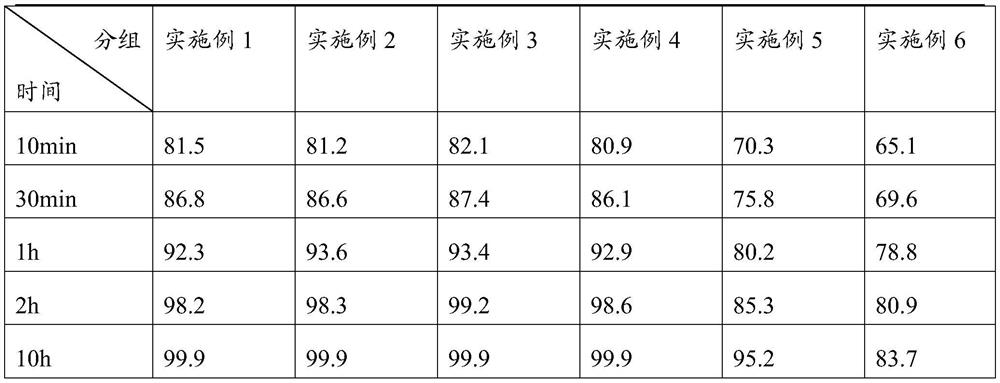
Embodiment 1
[0089] Add 3-ethyl-4-methyl-2-pyrrolidone into n-heptane, the mass ratio of 3-ethyl-4-methyl-2-pyrrolidone to n-heptane is 1:10~18, heat up to 70°C Dissolve, add phenylethyl isocyanate dropwise, the molar ratio of phenylethyl isocyanate to 3-ethyl-4-methyl-2-pyrrolidone is 1:1, heat to 100°C, react for 8 hours, and drop to At room temperature, the crude product of Compound A was obtained, and then methyl tert-butyl ether with the same mass as n-heptane was added, stirred for 25 minutes, filtered, and dried to obtain the pure product of Compound A;
[0090] Add pure compound A to chlorosulfonic acid, the mass ratio of chlorosulfonic acid to 3-ethyl-4-methyl-2-pyrrolidone is 2.5:1, the reaction temperature is 5°C, stir for 3.5h, add 5°C water, and crystal, filtered to obtain compound B;
[0091] Add compound B to saturated ammonia water, the solid-to-liquid ratio of saturated ammonia water to 3-ethyl-4-methyl-2-pyrrolidone is 1g:18ml, the reaction temperature is 35°C, stirred f...
Embodiment 2
[0097] Add 3-ethyl-4-methyl-2-pyrrolidone into n-heptane, the mass ratio of 3-ethyl-4-methyl-2-pyrrolidone to n-heptane is 1:12, heat up to 85°C to dissolve, Add phenylethyl isocyanate dropwise, the molar ratio of phenylethyl isocyanate to 3-ethyl-4-methyl-2-pyrrolidone is 1:1.2, heat to 90°C, react for 10h, and cool down to room temperature after completion, To obtain the crude product of Compound A, add methyl tert-butyl ether equal in quality to n-heptane, stir for 20 min, filter, and dry to obtain the pure product of Compound A;
[0098] Add pure compound A to chlorosulfonic acid, the mass ratio of chlorosulfonic acid to 3-ethyl-4-methyl-2-pyrrolidone is 2.8:1, the reaction temperature is 10°C, stir for 3h, add 10°C water, crystallize , filtered to obtain compound B;
[0099] Add compound B to saturated ammonia water, the solid-to-liquid ratio of saturated ammonia water to 3-ethyl-4-methyl-2-pyrrolidone is 1g:15ml, the reaction temperature is 30°C, stirred for 2.5h, filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com