Refining method of glimepiride bulk drug
A refining method and raw material medicine technology, applied in organic chemistry and other fields, can solve problems such as low purification efficiency and increased content of impurity III, and achieve the effects of improving purification efficiency, mild process conditions, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for refining a glimepiride bulk drug, comprising the following steps:
[0042] Stir 10 g of crude glimepiride into 600 g of purified water, raise the temperature to 65°C, stir and dissolve until the solution is clear, and filter while hot. Cool the filtrate to 30°C, adjust the pH to 2 with 2mol / L hydrochloric acid to obtain a slurry, keep stirring for 1 hour, centrifuge, filter to obtain a filter cake, rinse with purified water until neutral, and control the water content of the filter cake to 25% .
[0043] Add the filter cake into a mixed solvent of 11 g of dichloromethane and 89 g of acetone, stir and heat up to reflux, filter while hot after 1 hour, rinse the filter cake with acetone, and drain it. Dried at 60°C to obtain 9.50 g of Glimepiride Fine Pharmaceutical.
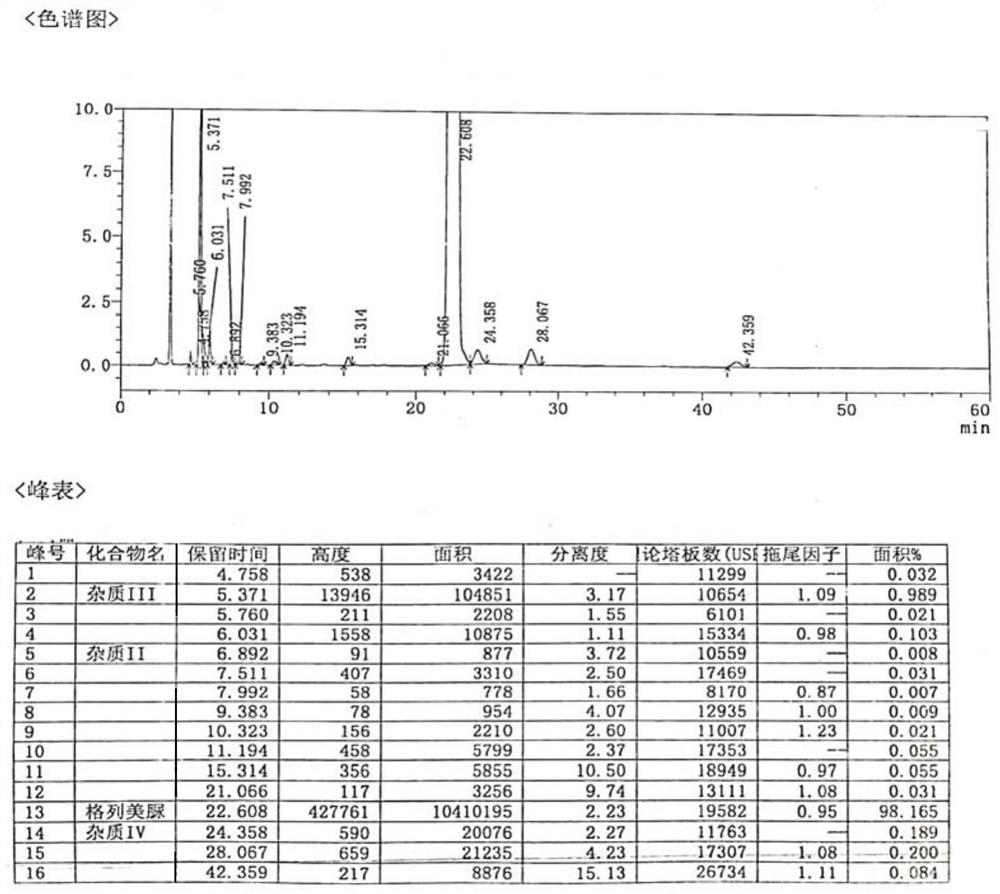
[0044] The purity of glimepiride refined pharmaceuticals measured by high performance liquid chromatography is 99.423%, and the content of impurity III is 0.237%, such as figure 2 .
Embodiment 2
[0046] Stir 10g of crude glimepiride into 800g of purified water, raise the temperature to 60°C, stir and dissolve until the solution is clear, filter while it is hot, cool the filtrate to 20°C, then adjust the pH to 5 with 3mol / L hydrochloric acid to obtain a slurry, keep it warm After stirring for 1 hour, centrifuge and filter to obtain a filter cake, rinse with purified water until neutral, and control the water content of the filter cake to 40%. Add the filter cake to a mixed solvent of 10 g of dichloromethane and 70 g of acetone, stir and heat up to reflux, filter while hot after 1 hour, rinse the filter cake with acetone, and drain it. Dry at 65°C to obtain 9.43 g of glimepiride.
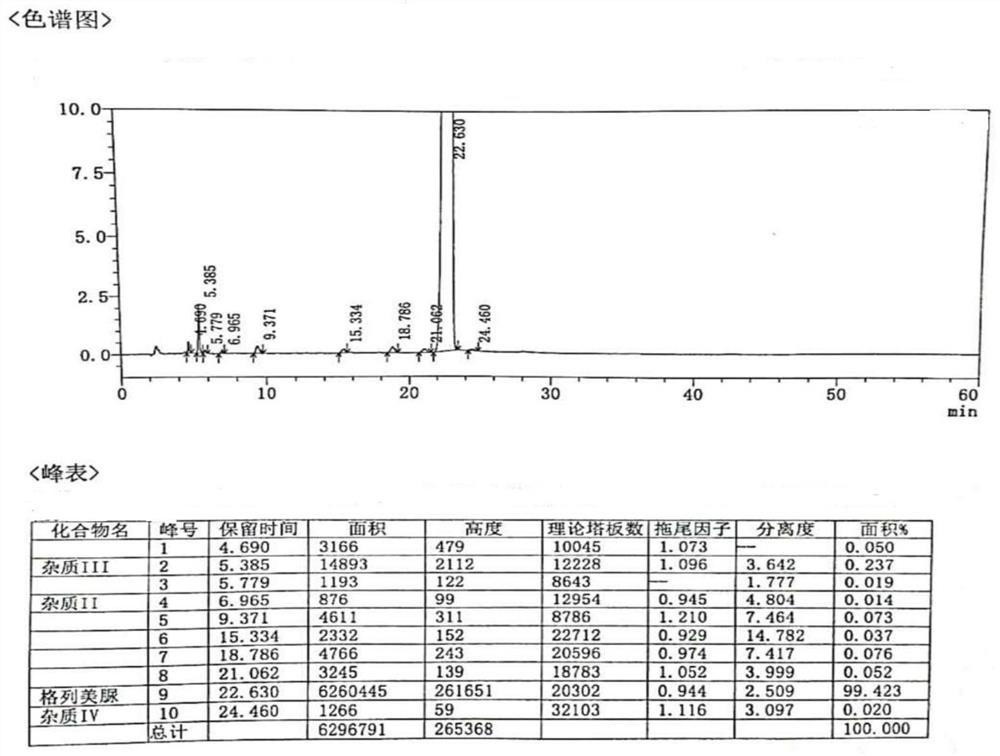
[0047] The purity of glimepiride refined medicine measured by high performance liquid chromatography is 99.537%, and the content of impurity III is 0.280%, such as image 3 .
Embodiment 3
[0049] Stir 20g of crude glimepiride into 1400g of purified water, raise the temperature to 70°C, stir and dissolve until the solution is clear, filter while it is hot, cool the filtrate to 25°C, adjust the pH to 3 with 1mol / L hydrochloric acid to obtain a slurry, keep stirring After 1 hour, centrifuge and filter to obtain a filter cake, rinse with purified water until neutral, and control the water content of the filter cake to 30%.
[0050]Add the filter cake into a mixed solvent of 20 g of dichloromethane and 160 g of acetone, stir and heat up to reflux, and filter while hot after reflux for 2 hours. The filter cake is rinsed with acetone and drained. Dry at 60°C to obtain 18.92 g of glimepiride.
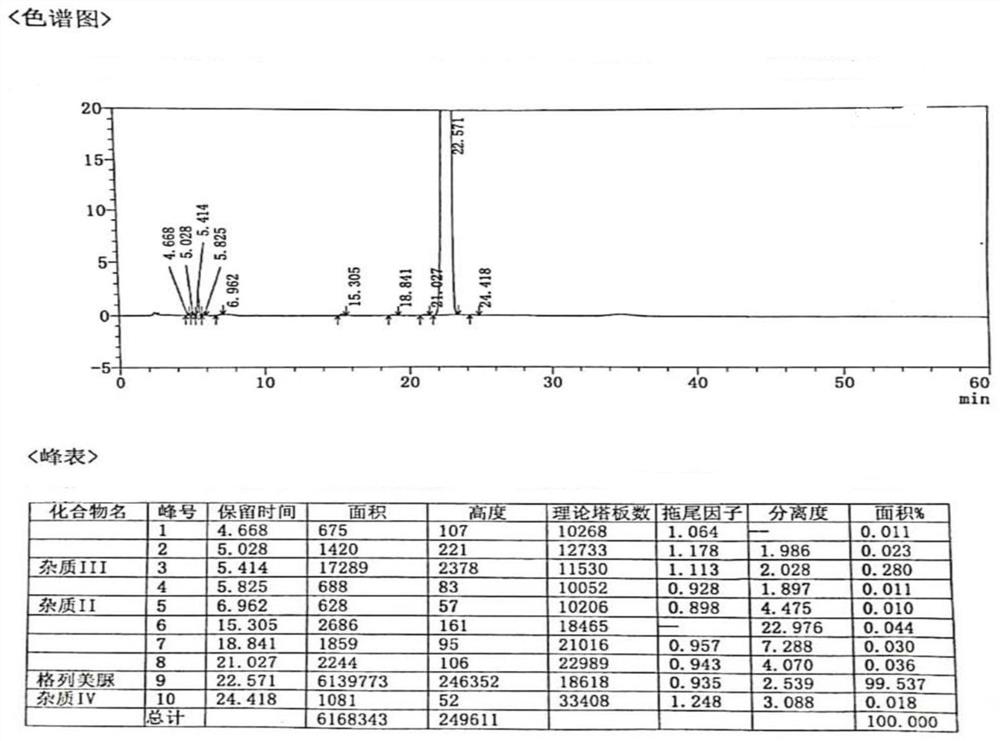
[0051] The purity of glimepiride refined pharmaceuticals measured by high performance liquid chromatography is 99.621%, and the content of impurity III is 0.211%, such as Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com