A kind of assay method of dissolution curve of coenzyme q10 capsule
A measurement method and capsule technology, applied in measuring devices, testing pharmaceutical preparations, instruments, etc., can solve problems such as inability to evaluate differences, and achieve the effect of quality assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] 1.1 Preparation of dissolution medium
[0041] Weigh an appropriate amount of Triton x-100 and polysorbate 80, add water to dissolve, dilute to 1000mL, and shake well.
[0042] 1.2 Chromatographic conditions:
[0043] Chromatographic column: Waters MicroBondapak C18 (4.6mm×150mm, 10μm) chromatographic column
[0044] Mobile phase: methanol: absolute ethanol = 65:35
[0045] Detection wavelength: 275nm
[0046] Flow rate: 1.0ml / min
[0047] Injection volume: 20μL;
[0048] Column temperature: 30°C;
[0049] Isocratic elution.
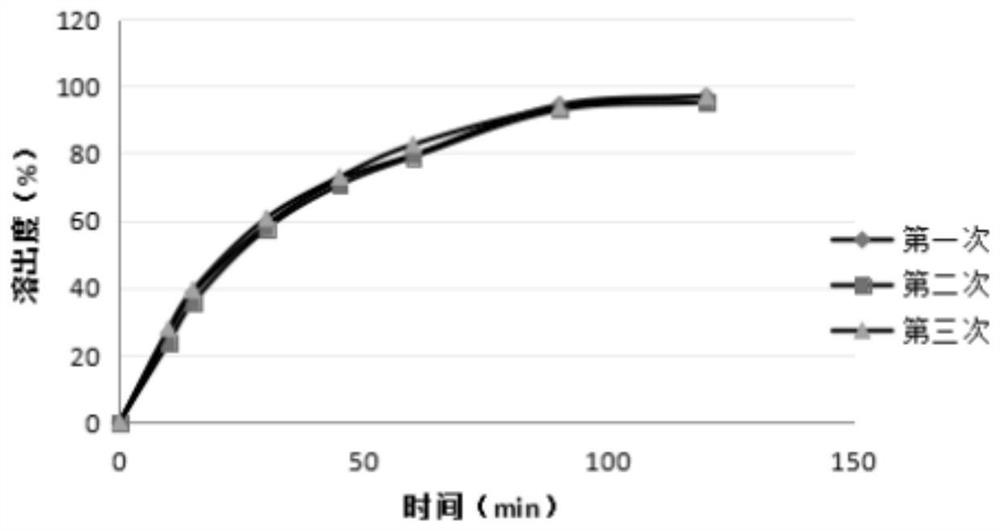
[0050] 1.3 Dissolution curve determination method
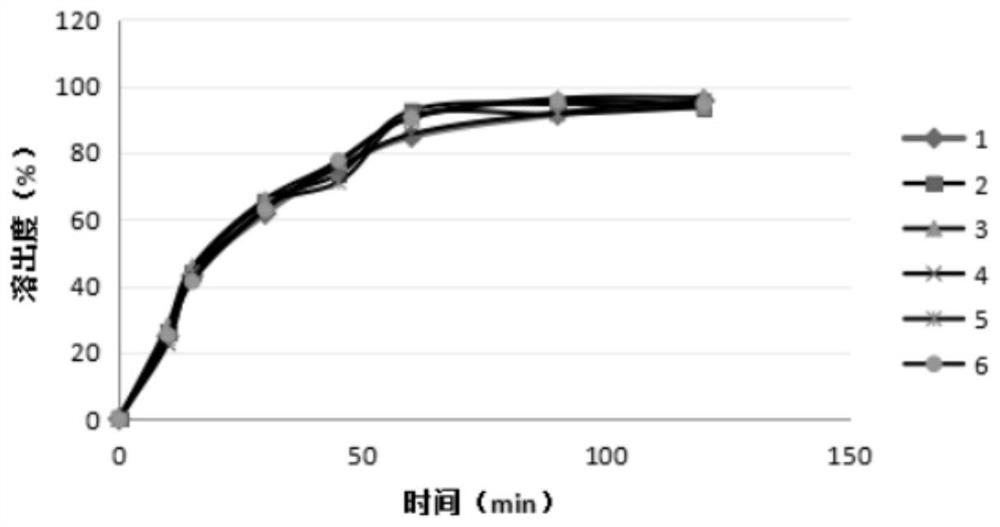
[0051] After taking the coenzyme Q10 capsules and packing them into the settling basket, adopt the paddle method (the paddle method refers to the dissolution determination method of the four general rules of the Pharmacopoeia 2015 edition), and mix the solutions A (5wt% Triton, water), B (5wt% Triton) respectively. through+0.1wt% polysorbate 80 aqueous solution), C (5wt% triton+0.2wt% polys...
Embodiment 1
[0057] A dissolution curve determination method of coenzyme Q10 capsules, the method specifically comprises the following steps:
[0058] Dissolution medium: mixed solution E (5wt% triton+1wt% polysorbate 80 aqueous solution).
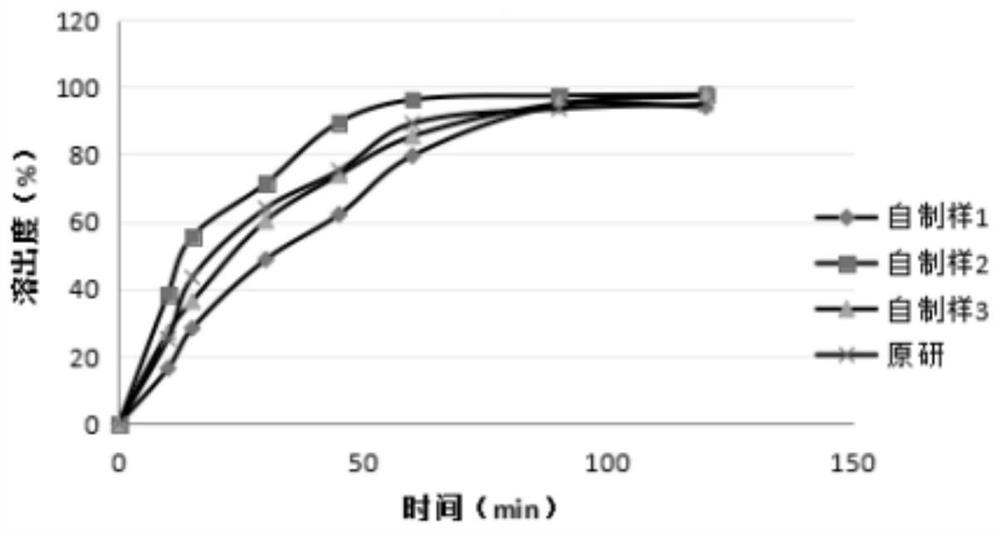
[0059] The dissolution curves of three batches of self-made samples and original research samples with different prescriptions (Table 2) were measured by the dissolution test method in 1.3. The difference in dissolution behavior between different prescriptions is obvious, indicating that this method has a strong distinguishing effect and is suitable for this application. The mensuration of capsule dissolution curve, concrete measurement result is as follows table 3, and dissolution curve sees attached figure 1 .
[0060] Table 2 Sample information
[0061] batch number Homemade sample 1 Q-20190601 Homemade sample 2 Q-20190703 Homemade Sample 3 Q-20190905 Original preparation 85A77S
[0062] Table 3 The result...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com