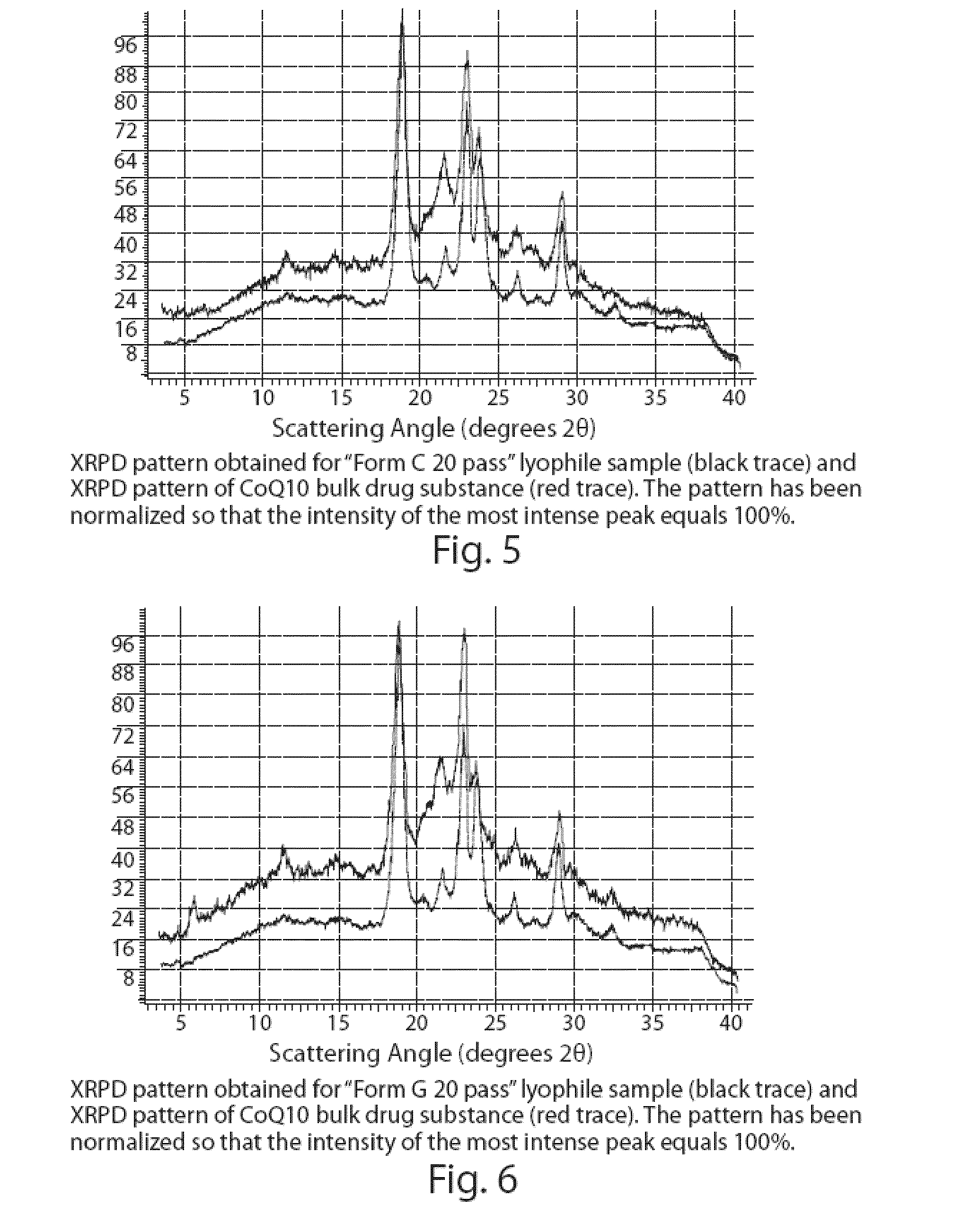
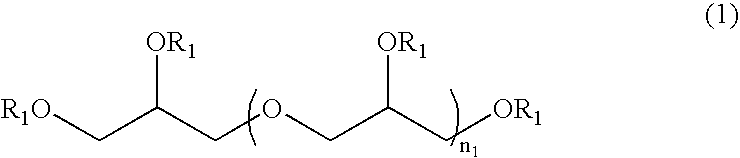
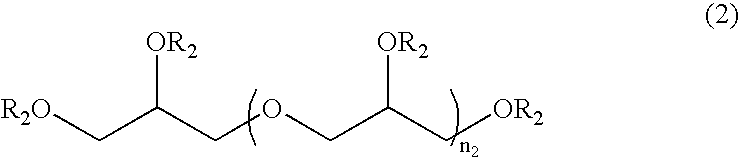
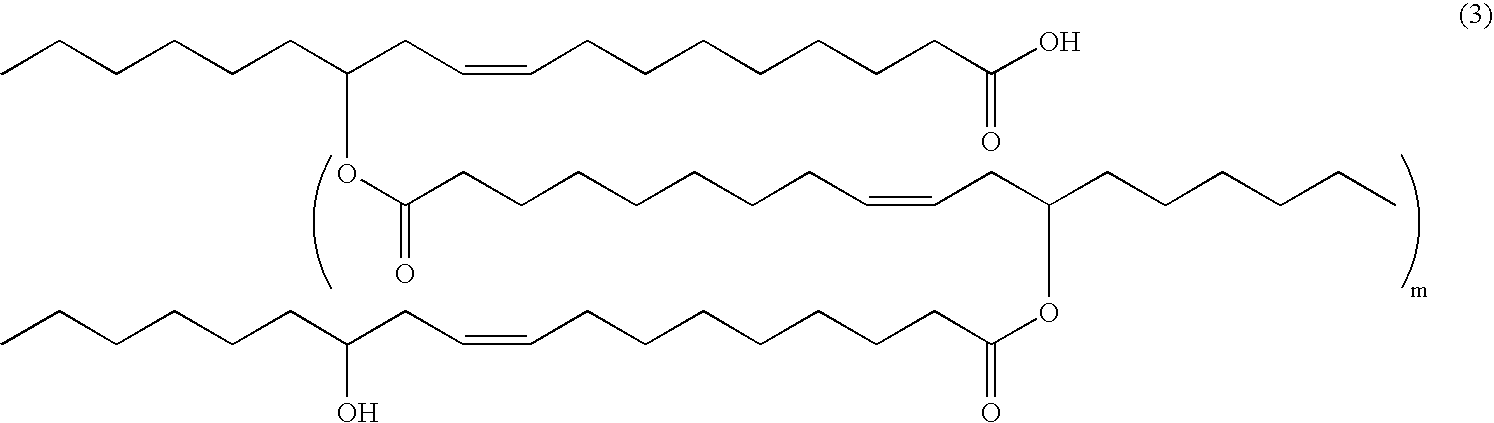
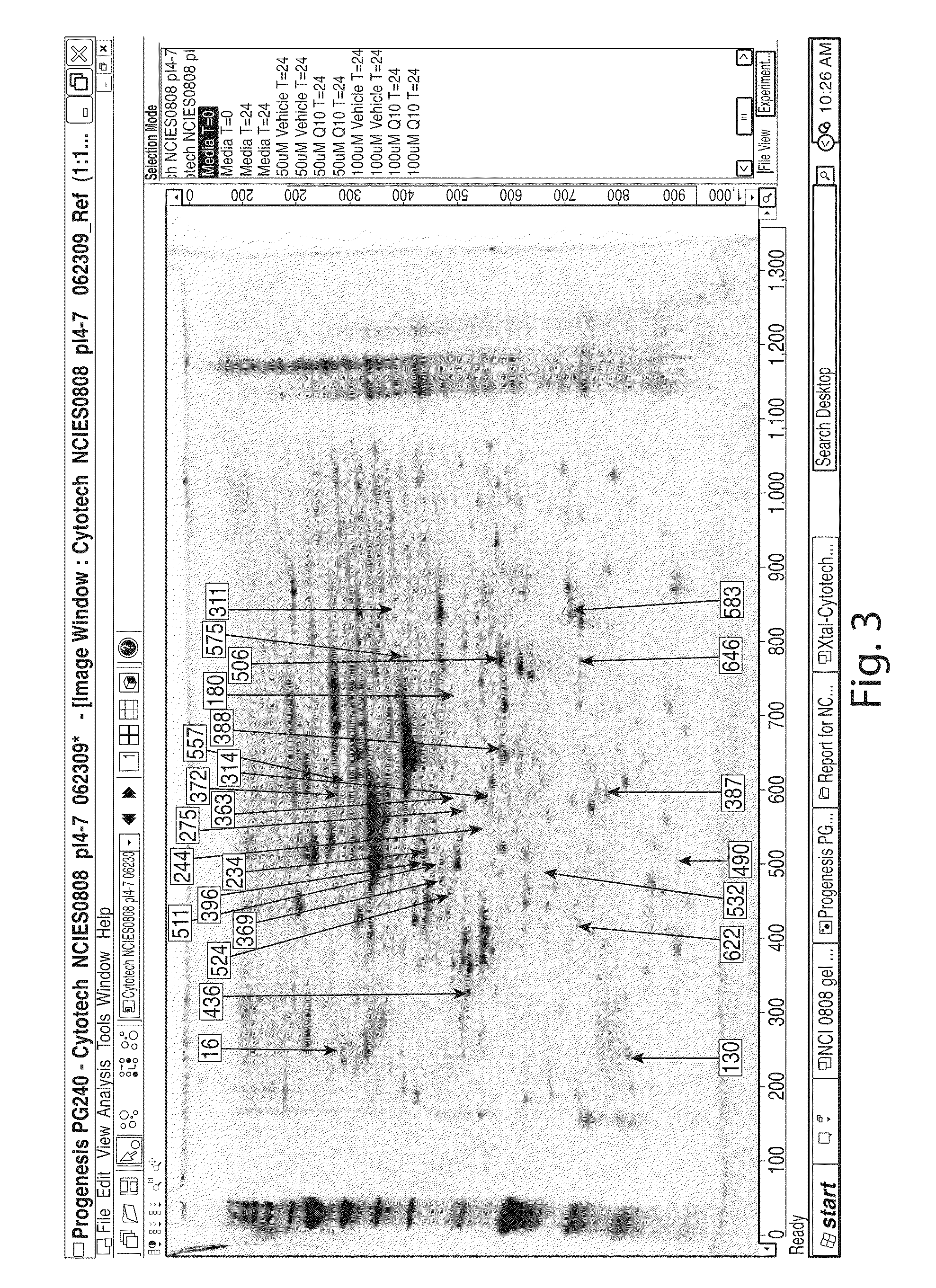
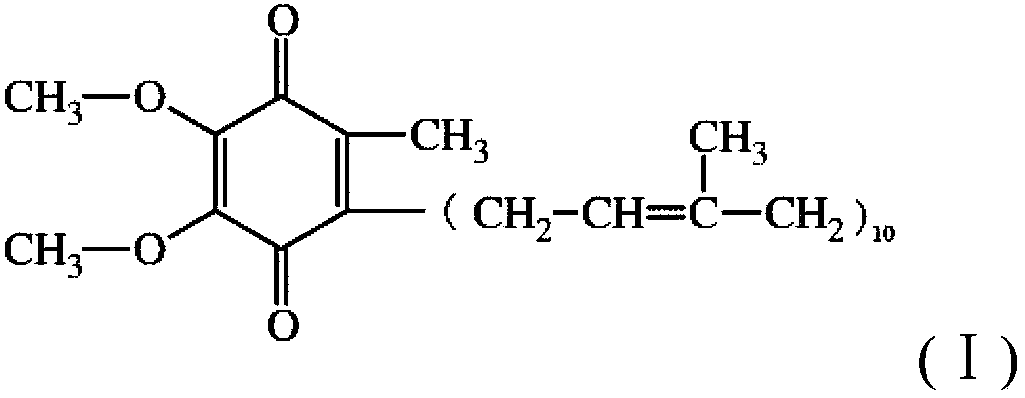
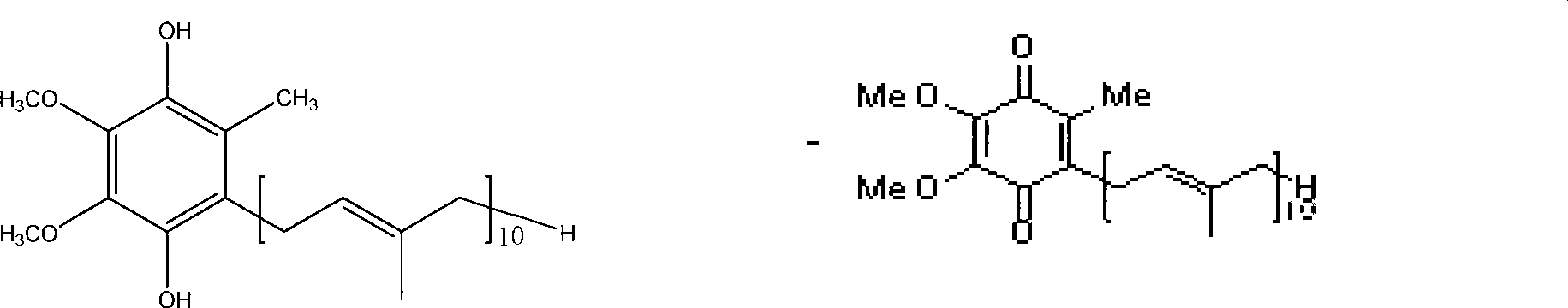Patents
Literature
510 results about "Coenzyme Q10" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coenzyme Q, also known as ubiquinone, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is Coenzyme Q₁₀ or ubiquinone-10. CoQ₁₀ is not approved by the U.S. Food and Drug Administration (FDA) for the treatment of any medical condition. It, however, is sold as a dietary supplement.
Composition Containing Statins and Omega-3 Fatty Acids
InactiveUS20080089876A1Hydroxy compound active ingredientsPeptide/protein ingredientsFatty acidStatine
A combination is described comprising at least one omega-3 fatty acid, optionally esterified or salified, at least one statin, Coenzyme Q10, resveratrol, at least one policosanol, pantethine, selenium, and zinc. This combination is endowed with a synergistic effect and is useful in the treatment of disease forms due to insulin resistance and in cardiovascular diseases.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Pharmaceutical composition and method for the transdermal delivery of calcium
InactiveUS20070292493A1Reduce disadvantagesReduce and prevent likelihoodHalogenated hydrocarbon active ingredientsBiocideArginineTryptophan
The present invention relates to a method and transdermal pharmaceutical composition for preventing or reducing the likelihood of calcium deficiency or imbalances caused by calcium deficiency. The transdermal pharmaceutical composition includes a therapeutically effective amount of a pharmaceutically acceptable salt of calcium and a pharmaceutically acceptable carrier constituting a pluronic lecithin organogel. In addition to calcium, the transdermal pharmaceutical composition may also contain a therapeutically effective amount of: (1) a pharmaceutically acceptably salt of other minerals such as magnesium, zinc, selenium, manganese, or chromium; (2) a vitamin such as vitamin A, vitamin D, vitamin C, vitamin E or B-complex vitamins, choline, lecithin, inositol, PABA, biotin, or bioflavomoids; (3) a carotenoid such as lycopene or lutein; (4) a hormone such as dehydroepiandrosterone, progesterone, pregnenolone, or melatonin; (5) an amino acid such as arginine, glutamine, lysine, phenylalanine, tyrosine, GABA, tryptophan, carnitine, or acetyl-l-carnitine; (6) a fatty acid such as a fish oil or flax seed oil; (7) a vita-nutrient such as coenzyme Q10; (8) a cartilage building nutrient such as glucosamine, chondroitin, or MSM, (9) a herb such as ginkgo biloba, echinacea, 5-HTP, St. John's wort, or saw palmetto; or (9) any combination thereof. The transdermal pharmaceutical composition may be topically administered to a human to prevent or reduce the likelihood of calcium deficiency or imbalances caused by calcium deficiency such as hypertension, high cholesterol, colon and rectal cancer, osteomalacia, rickets, osteoporosis, cardiovascular disease, preeclampsia, tooth decay, and premenstrual syndrome.
Owner:BRIERRE BARBARA T
Self emulsifying compositions for delivering lipophilic coenzyme Q10 and other dietary ingredients
InactiveUS20060051462A1Oral administration is convenientIncrease loadOrganic active ingredientsFood ingredientsSolubilityDietary supplement
The present invention provides novel dietary supplement compositions based on the use of a particular oil phase which comprises of Coenzyme Q10 and optionally other lipophilic dietary ingredients of low water solubility and a liquid mixture which comprises one or more emulsifiers, a fatty acid monoester formed between an short chain alcohol of C1 to C4 chain length and a saturated, or mono-unsaturated, or di-unsaturated (both conjugated and non-conjugated) fatty acid of C6 to C24 chain length, or medium chain mono- / di-esters, or the mixture of above. The composition is in a form of self-emulsifiable in the aqueous medium, for example, a simulated gastric fluid, which should provide a high oral bioavailability for the lipophilic dietary ingredients.
Owner:WANG JIMMY X
Topical management of ocular and periocular conditions
Chronic glaucoma, cataract, ocular and periocular aging are treated and prevented by the administration of agents that affect metabolic subsystems such as (i) mitochondrial bioenergetics, (ii) free radical moderation and glutathione maintenance, (iii) constitutive nitric oxide / endothelin-1 balance, and (iv) calcium wave signaling and associated neuronal excito-toxicity. Included among the agents are tetrahydrobiopterin, R-alpha-lipoic acid, coenzyme Q10, 17 alpha-estradiol, and glutathione.
Owner:CHRONORX
External skin care product capable of adjusting skin immunity and delaying skin aging
ActiveCN103520081ARegulate immunityDelay agingCosmetic preparationsToilet preparationsBiotechnologyCentella asiatica extract
The invention discloses an external skin care product capable of adjusting skin immunity and delaying skin aging. The external skin care product is characterized by comprising powder, an aqueous solution and emulsion, wherein the powder comprises oligopeptide-1 and ginsenoside; the aqueous solution comprises glycyrrhiza glabra root extract, artemisia capillaris flower extract, mulberry root extract, jujube fruit extract, scutellaria root extract, hydrolyzed rice protein and nicotinamide; and the emulsion comprises hgytantriol bifida ferment lysate, creatine, carnosine, alpha glucosyl hesperidin, hexapeptide-3, centella asiatica extract, coenzyme Q10 inclusion complex, opuntia ficus-indica stem extract, rhodiola rosea extract, saussurea involucrate extract, pseudo-ginseng root extract and Chinese angelica extract inclusion complex. According to the external skin care product, the powder, the aqueous solution and the emulsion are jointly used, and the external skin care product can reduce wrinkle and pachulosis, even skin tone and improve skin elasticity and skin firmness, so that the skin is finer and smoother and looks young.
Owner:INFINITUS (CHINA) CO LTD
Method and composition for improving fertility health in female and male animals and humans
InactiveUS7045151B2Increasing for conceptionFree from damageHeavy metal active ingredientsBiocideToxicantArginine
In a new pharmaceutical combination, the herb, Vitex agnus-castus (chasteberry), enhances hormone balance by increasing progesterone release and, therefore, ovulation frequency. The antioxidants, green tea, vitamin E, and selenium, improve overall reproductive health. L-arginine, an amino acid, stimulates the reproductive organs by improving circulation. Folic acid, vitamins B6 and B12, iron, zinc and magnesium help promote womens' fertility. Sperms are highly susceptible to free radical or oxidative damage from environmental toxicants and natural aging. Vitamins C and E, coenzyme Q10 and selenium are all potent antioxidants that help improve sperm counts and quality. Ferulic acid, an antioxidant found in Dong quai, also improves sperm quality. Zinc and B vitamins (B6, B12 and folate) are critical nutrients in male reproductive systems for hormone metabolism, sperm formation and motility. The amino acid, L-carnitine, promotes formation of healthy sperm.
Owner:THE DAILY WELLNESS
Compositions for the skin comprising fibers and ubiquinones and methods of using the same
The present invention relates to a composition for topical application on the skin, comprising fibers and at least one or two or more compounds selected from the group of the ubiquinones and derivatives thereof. The ubiquinone can in particular be coenzyme Q10. The composition of the invention makes it possible to improve the appearance of the skin, immediately as well as in the longer term, and to mask the imperfections and / or signs of ageing of the skin while maintaining a natural appearance of the skin.
Owner:LOREAL SA
INTRAVENOUS FORMULATIONS OF COENZYME Q10 (CoQ10) AND METHODS OF USE THEREOF
Disclosed herein are formulations suitable for parenteral administration of certain hydrophobic active agents such as Coenzyme Q10. Methods of preparing the same and methods of treatment of oncological disorders using the same are also provided herein. The formulations comprise an aqueous solution; a hydrophobic active agent dispersed to form a colloidal nano-dispersion of particles; and at least one of a dispersion stabilizing agent and an opsonization reducer wherein the colloidal nano-dispersion of the active agent is dispersed into nano-dispersion particles having a mean size of less than 200-nm. Methods of preparing the parenteral formulations comprise dispersing the hydrophobic active agent by high pressure homogenization by (1) adding hydrophobic active agent to a 65° C. bath of water and mixing to form a hydrophobic active agent / water mixture; (2) adding a dispersion stabilizing agent to the hydrophobic active agent / water mixture and mix at 65° C. to form a hydrophobic active agent / water / stabilizer mixture; (3) adding an opsonization reducer to form a hydrophobic active agent / water / stabilizer / reducer mixture; (4) pre-heating a Microfluidizer to 65° C.; and (5) processing by mixing the hydrophobic active agent / water / stabilizer / reducer mixture in the Microfluidizer at 65° C. such that a hydrophobic active agent colloidal nano-dispersion having a mean particle size less than 200-nm is formed. Provided herein are also methods of treating oncological disorders by administering formulations described herein to a subject such that treatment or prevention of the oncological disorder occurs.
Owner:BPGBIO INC
Compositions containing reduced coenzyme Q10 and carotenoid
InactiveUS20050226858A1High activityExhibit activityBiocidePeptide/protein ingredientsOxygenCoenzyme Q10
A composition having an antioxidative activity, especially, having a sufficient activity for removing active oxygen and / or free radicals is provided. The composition includes both reduced coenzyme Q10 and a carotenoid, and the activity for removing active oxygen and / or free radicals is synergistically enhanced. A method for removing active oxygen and / or free radicals by using the composition containing both reduced coenzyme Q10 and a carotenoid is also provided.
Owner:KANEKA CORP
Method for stabilizing reduced coenzyme Q10
ActiveUS20070104701A1Stable storageIncrease residual ratioPeptide/protein ingredientsCapsule deliveryGlycerolCompound (substance)
The present invention has its object to provide a method for stabilizing reduced coenzyme Q10, which is usable as foods, functional nutritive foods, specific health foods, nutritional supplements, nutrients, animal drugs, drinks, feeds, cosmetics, medicines, remedies, preventive drugs and the like. Reduced coenzyme Q10, which is readily oxidized in the air, is stabilized by causing ascorbic acid or a related compound thereof to coexist with a polyglycerol fatty acid ester with a polymerization degree of glycerol being not lower than 3 and / or a condensed ricinoleic acid polyglyceride in a mixture of the reduced coenzyme Q10 and an oil and fat.
Owner:KANEKA CORP
Toothpaste containing coenzyme Q10 and preparation method thereof
ActiveCN101502482APrevent atrophyImprove organizational vitalityCosmetic preparationsToilet preparationsFoaming agentOral ulcers
The invention relates to a toothpaste containing coenzyme Q10 and a preparation method thereof, in particular to a composition of toothpastes, belonging to the field of oral healthcare products; based on weight percentage, the coenzyme Q10 in the toothpaste is 1-2% and Chinese herbal extract is 0.5-2%; the rest of accessories are conventional materials for preparing toothpastes. The formulation proportion of the components in the toothpaste is preferably as follows: 2% of coenzyme Q10 mixture, 0.7% of Chinese herbal extract, 35% of abradant, 1.5% of bonding agent, 35% of wetting agent, 0.2% of flavoring agent, 2.5% of foaming agent, 1.5% of essence and 21.6% of deionized water. The toothpaste containing coenzyme Q10 has the healthcare functions the same as the common toothpastes, such as brightening and cleaning the teeth, stopping bleeding of gums and healing oral ulcer; besides, the coenzyme Q10 and Chinese herbal extract permeate the gum cell membrane so that the gum cell obtains energy and tissue vigor is strengthened, thereby essentially conserving the gums, preventing periodontitis, gingivitis and gingival atrophy to reach the aim of strengthening the gums and fixing teeth.
Owner:玉溪健坤生物药业有限公司
Whole Mouth Malador Control By A Combination Of Antibacterial And Deodorizing Agents
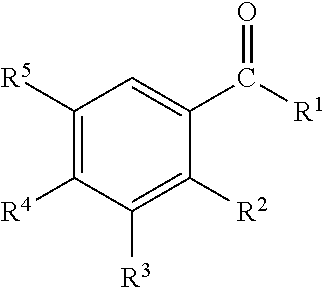
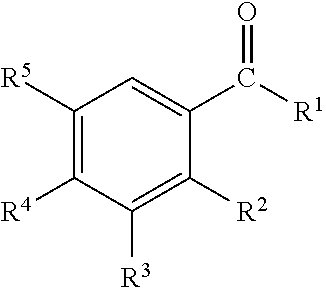
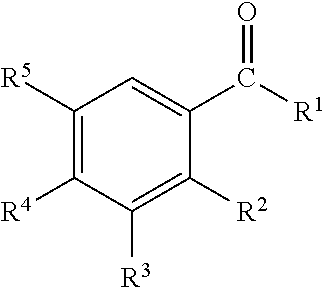
InactiveUS20110239736A1Effective controlCosmetic preparationsToilet preparationsTriclosanAmmonium compounds
Disclosed are oral care compositions effective to control mouth and breath malodour comprising in a pharmaceutically acceptable carrier, a combination of an antibacterial agent and a deodorizing or odor-neutralizing agent comprising a compound having the structurewherein R1, R2, R3, and R5 may be identical or different, each representing H, a linear or branched C1-C6 alkyl or alkenyl, phenyl, —OH or —ORa; R4 is —OH, —ORa, phenyl, or a linear or branched C1-C6 alkyl or alkenyl; and Ra is phenyl or a linear or branched C1-C6 alkyl or alkenyl. The odor-neutralizing agent may further comprise one or more of an additional odor-neutralizing compound selected from α-damascenone, α-isomethylionone, α-ionone, β-ionone, pulegone, piperitone, carvone, coenzyme Q10 or cinnamaldehyde.The antibacterial agent may comprise one or a mixture of a quaternary ammonium compound selected from cetylpyridinium chloride, tetradecylpyridinium chloride, N-tetradecyl-4-ethyl pyridinium chloride or domiphen bromide; metal ions such as stannous, zinc or copper; chlorhexidine; triclosan; triclosan monophosphate; or selected essential oils.
Owner:THE PROCTER & GAMBLE COMPANY
Composition containing reduced coenzyme Q10 and production method thereof
ActiveUS20080026063A1Promote oral absorptionPromote absorptionBiocidePowder deliveryBiotechnologyDietary supplement
The present invention provides a particulate composition wherein an oil component containing reduced coenzyme Q10 is polydispersed forming a domain in a matrix containing a water-soluble excipient, which simultaneously shows high oxidative stability and high oral absorbability, a production method thereof, and a stabilizing method thereof. It also provides a food, food with nutrient function claims, food for specified health uses, dietary supplement, nutritional product, animal drug, drink, feed, pet food, cosmetic, pharmaceutical product, therapeutic drug, prophylactic drug and the like, which contain the composition.
Owner:KANEKA CORP
Methods for treatment of human skin damaged by laser treatment or chemical peelings and compositions useful in such methods
InactiveUS20030012762A1Eliminate disadvantageEliminate disadvantagesCosmetic preparationsBiocideCompound (substance)Co enzyme q10
Methods for treatment of human skin damaged by laser treatment or chemical peelings, said method comprising topical application of a lamellar oil-in-water system, said lamellar oil-in-water system comprising at least one vegetable oil as the oily component and at least one hydrogenated phospholipid as emulsifier, said at least one hydrogenated phospholipid comprising not more than 10 percent by weight of negatively charged phospholipids the remainder being neutral phospholipids. Compositions useful in carrying out said methods are lamellar oil-in-water systems comprising at least one vegetable oil as the oily component and at least one hydrogenated phospholipid as emulsifier, said at least one hydrogenated phospholipid comprising not more than 10 percent by weight of negatively charged phospholipids the remainder being neutral phospholipids. Said lamellar oil-in-water system may further comprises sodium carboxymethyl beta-glucan (Sodium Carboxymethyl Betaglucan) as a compound for improving wound healing and / or coenzyme Q10 as a compound for improving skin regeneration.
Owner:MIBELLE COSMETICS
Coenzyme q10 containing proliposome and preparation thereof
InactiveUS20060251708A1Improve stabilityMixing of flexible and convenientCosmetic preparationsPeptide/protein ingredientsLipid formationSpray dried
A coenzyme Q10 and ceramide-containing preliposome, and pharmaceutical preparations and cosmetics containing the same. The preliposome can further comprise other lipid components. A granular and lyophilized solid-preparation is produced by lyophilization or spray drying. Then, the coenzyme Q10-containing proliposome is obtained by adding water in the said solid-preparation and shaking. Transdermal absorption of coenzyme is improved together with the effect of coenzyme Q10 in cosmetics and the stability of coenzyme Q10 and liposome, which facilitate the formulation of cosmetics.
Owner:SHANGHAI JAHWA UNITED
Method for extracting coenzyme Q10 from microorganism
ActiveCN101381747AEasy to operateEasy extractionOrganic chemistryMicroorganism based processesBiotechnologyMicroorganism
The invention discloses a method for extracting coenzyme Q10 from microorganisms, wherein the ultrasonic disintegration is to mix microorganisms with the ability of producing the coenzyme Q10 and an organic solvent in which the coenzyme Q10 is well dissolved according to the mass volume ratio of 1-5 to 10-20, and to obtain the coenzyme Q10 after ultrasonication at a temperature of 0 DEG C, wherein the ultrasonic frequency is between 0.2 and 0.8 and the power is between 300 and 500 watts; and the alkaline splitting method is to extract the coenzyme Q10 from fermentation broth of Rhodobacter sphaeroides. The microorganisms are preferred to be microorganisms of the Rhodobacter sphaeroides. The method for extracting the coenzyme Q10 has the advantages of high efficiency, low pollution, low requirements on apparatuses and equipment, low cost and so on, and is suitable for promotion and application.
Owner:FIELD OPERATION BLOOD TRANSFUSION INST OF PLA SCI ACAD OF MILITARY
Coenzyme Q10 oral emulsion and preparing process thereof
InactiveCN101015524AImprove stabilityEasy to takeOrganic active ingredientsAntinoxious agentsPurified waterAnti oxidant
The invention relates to a coenzyme Q10 oral emulsion and its preparation method, wherein the constituents include coenzyme Q10 0.1-80%, medicinal oil 1-95%, emulsifying agent 0.5-30%, auxiliary emulsifying agent 0-10%, anti-oxidant 0.001-15%, and balancing purified water. The emulsion can be prepared through inversion phase emulsification method, PIT emulsification method, alternative liquid feeding emulsification method, or continuous emulsification method.
Owner:SHENYANG PHARMA UNIVERSITY
Coenzyme Q10-containing fine particle with excellent dispersibility
InactiveUS20070053985A1Simple and easy mannerHandleability and transporting abilityBiocidePowder deliveryMicroparticleLotion
The present invention has an object to provide coenzyme Q10-containing particles, which can be easily and simply dispersed into water without any surfactants or other additives, and their production method. The present invention makes it easier to prepare an aqueous dispersion containing coenzyme Q10, which is hard to disperse in water, as fine particles by modifying coenzyme Q10 into a coenzyme Q10-containing fine particle comprising coenzyme Q10 covered with a biocompatible polymer. Therefore, compositions containing dispersed coenzyme Q10 in water, such as drinkable preparations, lotions, etc., can be produced in easy and simple manners.
Owner:KANEKA CORP
Method and composition for treating oral bacteria and inflammation
ActiveUS20050196359A1Growth inhibitionReduce inflammationCosmetic preparationsBiocideMaxillary gingivaBacterial growth
A composition and method for treating periodontal infections and gingivitis includes an extract of Centipeda cunninghami, coenzyme Q10, aloe vera and folic acid. The composition also contains additional plant extracts and nutrients that are effective in cell reproduction, wound healing and provide antibacterial and anti-inflammatory effects. The composition is applied to the teeth and gums to inhibit bacterial growth and reduce inflammation of the gums.
Owner:BIO BOTANICA
Formulation and manufacturing process for Coenzyme Q10 soft gel capsules
InactiveUS20050037066A1Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsManufacturing technologyMedicine
A soft gelatine capsule formulation improved manufacturing of Coenzyme Q10, comprising Coenzyme Q10 in a thixotropic gelatine carrier capable of admixing without heating with Coenzyme Q10, and capable of keeping Coenzyme Q10 in suspension at ambient temperature.
Owner:SOFT GEL TECHNOLGIES
Method for preparing high-purity coenzyme Q10 in large scale
ActiveCN102391092AReduce manufacturing costImprove product qualityQuinone separation/purificationBulk chemical productionAlcohol ethylPhysical chemistry
The invention relates to a method for preparing a separation coenzyme Q10, and in particular relates to a method for preparing a high-purity coenzyme Q10 in a large scale. The method comprises the following steps of: carrying out supercritical extraction on dry thallus containing the coenzyme Q10 to obtain extract liquor; and carrying out alkali treatment on the extract liquor to obtain a column loading liquid, carrying out column chromatography on the column loading liquid to obtain an eluent, concentrating the eluent, and crystallizing with ethanol to obtain the high-purity coenzyme Q10 with the purity higher than 99.5%. When the method provided by the invention is used for producing the coenzyme Q10, the overall yield is higher than 90%, and cost is low, thus the method provided by theinvention is applicable to industrial production.
Owner:杭州华东医药集团康润制药有限公司
Skin care composition and application thereof
InactiveCN103271839ADelay agingGood skin careCosmetic preparationsToilet preparationsMedicineCoenzyme Q10
The invention discloses a skin care composition, wherein the composition mainly comprises the following materials according to parts by weight: 0.1-5 parts of L-fucose, 0.05-5 parts of coenzyme Q10, 0.1-5 parts of hyaluronic acid, 0.05-2 parts of ceramide, and 1-25 parts of mPEG. The skin care composition can be prepared into any common skin care preparation. In addition, the invention further discloses application of the composition in preparation of the skin care composition having the functions of preventing aging and skin inflammation and moisturizing.
Owner:西宝生物科技(上海)股份有限公司
Formulation and manufacturing process for Coenzyme Q10 soft gel capsules
InactiveUS20050031681A1Improve solubility and stabilityPromote absorptionBiocidePeptide/protein ingredientsManufacturing technologyMedicine
A soft gelatine capsule formulation improved manufacturing of Coenzyme Q10, comprising Coenzyme Q10 in a thixotropic gelatine carrier capable of admixing without heating with Coenzyme Q10, and capable of keeping Coenzyme Q10 in suspension at ambient temperature.
Owner:SOFT GEL TECHNOLGIES
Method for fermentation preparation of cozymase Q10
The invention relates to a method for producing coenzyme Q10, which comprises the following steps: firstly, high density fermentation is performed to true alcaligenes eutruphus till the dry weight of thallus is above 90 gram / liter; secondly, the thallus is separated from a fermentation liquid; thirdly, the coenzyme Q10 is extracted from the separated thallus. The invention also relates to an application of true alcaligenes eutruphus in the coenzyme Q10 produced through the high density fermentation.
Owner:TIANAN BIOLOGIC MATERIAL NINGBO
Method of stabilizing reduced coenzyme q10
ActiveUS20050008630A1Quality improvementMinimize formationOrganic active ingredientsEther separation/purificationCompound (substance)Coenzyme Q10
The present invention provides a stabilization method, a preservation method and the like method of reduced coenzyme Q10, which is useful as functional nutritive foods, specific health foods and the like. Furthermore, the present invention provides a method for efficiently obtaining reduced coenzyme Q10 of high quality and by a method suitable for a commercial production. It is possible to handle and stably preserve reduced coenzyme Q10 under a condition that oxidation by a molecular oxygen is inhibited by contacting reduced coenzyme Q10 with an ascorbic acid and citric acid or a related compound thereof, and thus a stabilized composition is obtained. Moreover, reduced coenzyme Q10 is converted into a crystalline state in such a condition that the formation of oxidized coenzyme Q10 as a byproduct is minimized by crystallizing reduced coenzyme Q10 in the presence of ascorbic acid or a related compound thereof, etc., and thus a reduced coenzyme Q10 crystal of high quality is produced. Furthermore, by successively crystallizing the generated reduced coenzyme Q10 in the presence of ascorbic acid or a related compound thereof after reducing oxidized coenzyme Q10 to reduced coenzyme Q10 using ascorbic acid or a related compound thereof, operations are simplified and minimized, and thus reduced coenzyme Q10 of high quality is produced.
Owner:KANEKA CORP
Stabilization method of reduced coenzyme q10
ActiveUS20070258966A1Improve stabilityPromote absorptionCosmetic preparationsHydroxy compound active ingredientsCoenzyme Q10Coenzyme A
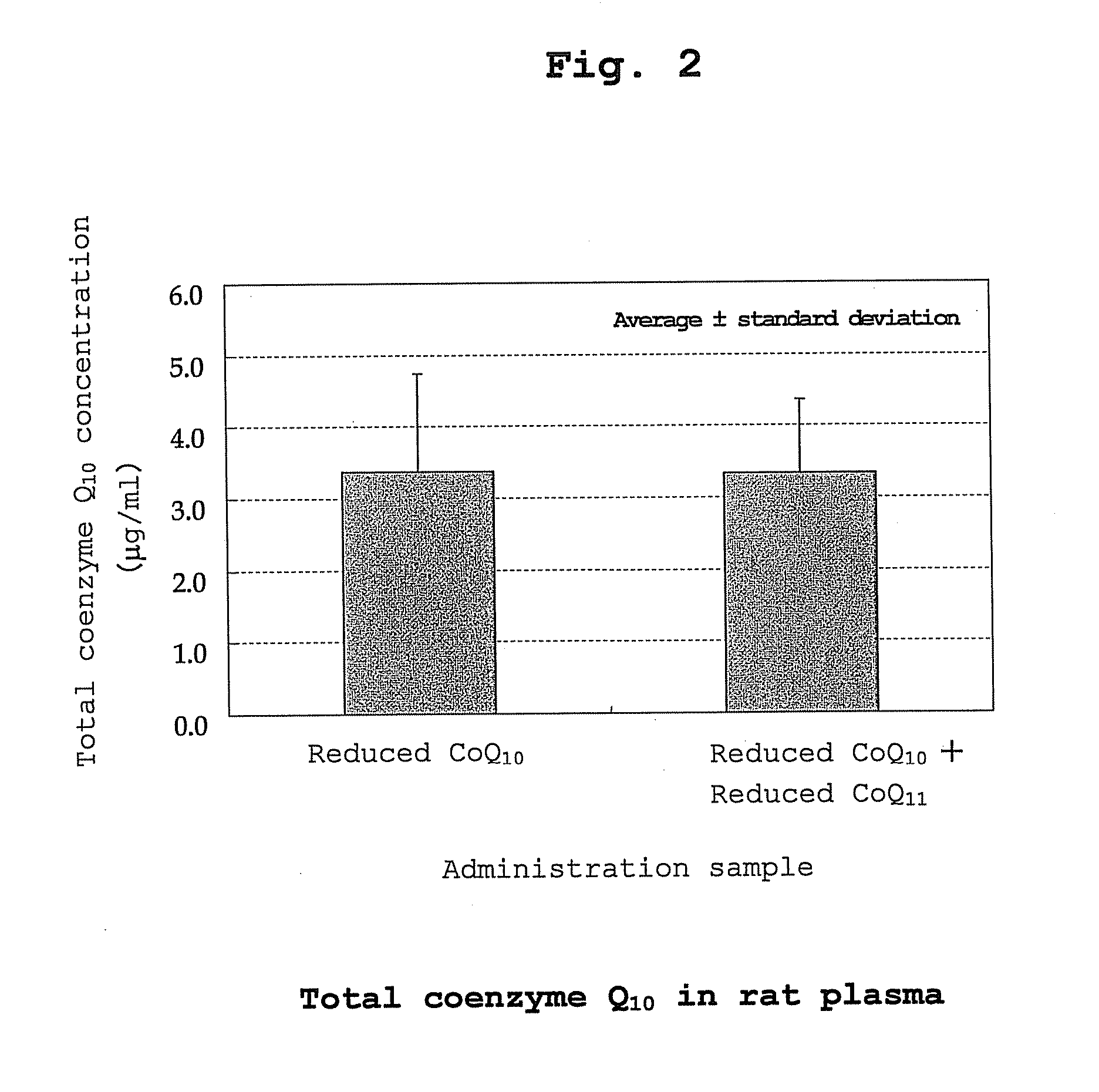
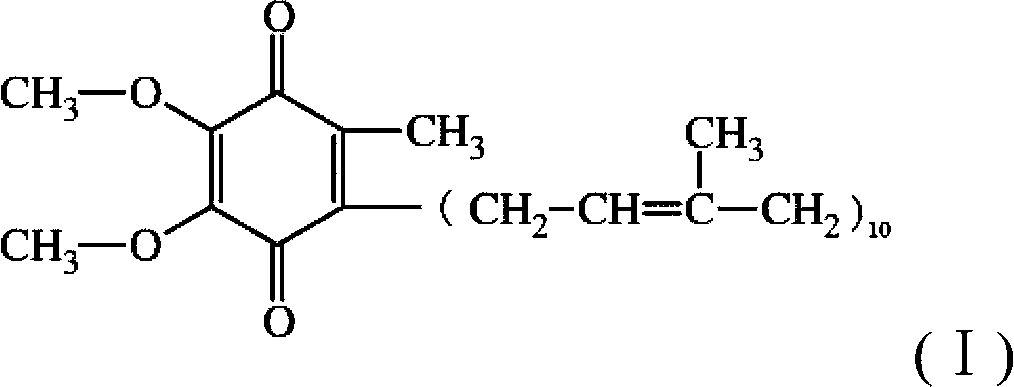
The present invention provides a method for stabilizing reduced coenzyme Q10, which is useful as a food, nutritional product, nutritional supplement, animal drug, drink, feed, cosmetic, pharmaceutical product, therapeutic drug, prophylactic drug and the like. The present invention also provides a method of producing a reduced coenzyme Q10-containing composition which includes the co-presence of reduced coenzyme Q10 and reduced coenzyme Q9 and / or reduced coenzyme Q11.
Owner:KANEKA CORP
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
InactiveUS20110129546A1Improve antioxidant capacityGood anti-inflammatory effectBiocideAntimycoticsDiseaseIsoflavones
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a base anti-inflammatory agent, such as indometacin; one or more optional active ingredients selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol™, L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF-α) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:UMBERT MILL IGNACIO
Coenzyme-Q10-production engineered bacteria construction method, engineered bacteria, and application thereof
ActiveCN103509816AIncrease productionReduce manufacturing costBacteriaMicroorganism based processesBiotechnologyBacteroides
The invention relates to the technical field of biology, and discloses a coenzyme-Q10-production engineered bacteria construction method, engineered bacteria, and an application thereof. The method comprises the steps that: a, total genomic DNA is extracted from Rhodobacter sphaeroides cacterial liquid; b, UbiG gene is obtained by amplification with a polymerase chain reaction; c, the amplified UbiG gene is connected with broad-host plasmid, such that recombinant vector is constructed; d, the recombinant vector is transferred to Escherichia coli S17-1; and e, the Escherichia coli S17-1 is subjected to conjugal transfer with the Rhodobacter sphaeroides, such that the engineered bacteria are obtained. According to the method provided by the invention for improving coenzyme Q10 yield through regulating Rhodobacter sphaeroides aromatic ring modification pathway, the operation is simple, and coenzyme Q10 synthesis capacity can be improved by higher than 30%. The method is suitable for coenzyme Q10 large-scale industrial production.
Owner:ZHEJIANG NHU CO LTD +1
Methods for treatment of a sarcoma using an epimetabolic shifter (coenzyme q10)
Methods and formulations for treating a sarcoma in humans using an epimetabolic shifter, such as Coenzyme Q10, a building block of CoQ10, a derivative of CoQ10, an analog of CoQ10, a metabolite of CoQ10, or an intermediate of the coenzyme biosynthesis pathway, are described. Methods for assessing the efficacy of treatment of, diagnosing, and prognosing sarcoma are also provided.
Owner:BERG
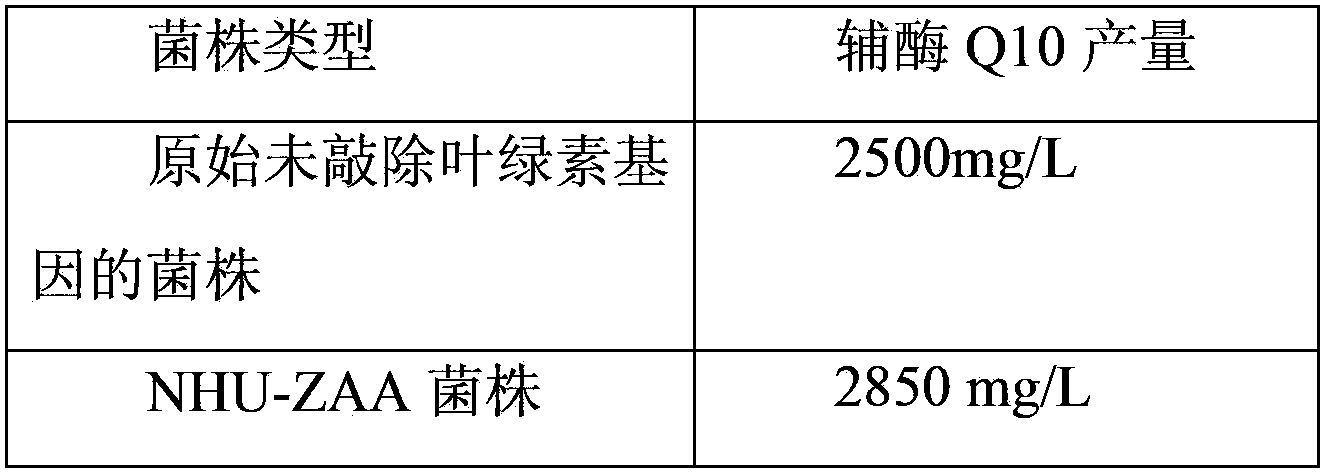
Coenzyme-Q10-production engineered bacteria construction method, engineered bacteria, and application thereof
ActiveCN103509728ALarge amount of synthesisIncrease productionBacteriaMicroorganism based processesEscherichia coliGenomic DNA
The invention relates to the technical field of biology, and discloses a coenzyme-Q10-production engineered bacteria construction method, engineered bacteria, and an application thereof. The method comprises the steps that: a, total genomic DNA is extracted from Rhodobacter sphaeroides cacterial liquid; b, bchG homologous gene is obtained by amplification with a polymerase chain reaction; c, the amplified homologous gene is connected with broad-host plasmid, such that recombinant vector is constructed; d, the recombinant vector is transferred to Escherichia coli S17-1; and e, the Escherichia coli S17-1 is subjected to conjugal transfer with the Rhodobacter sphaeroides, such that the engineered bacteria with chlorophyll synthesis gene bchG knocked out are obtained. According to the method provided by the invention for improving coenzyme Q10 yield through knocking out Rhodobacter sphaeroides chlorophyll synthesis gene, coenzyme Q10 synthesis capacity can be improved by higher than approximately 15%. The method is suitable for coenzyme Q10 large-scale industrial production.
Owner:ZHEJIANG NHU CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com