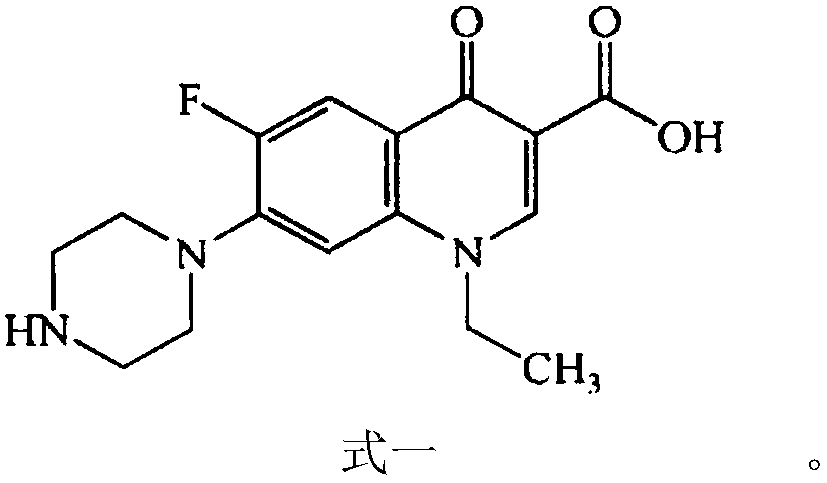
Oral norfloxacin capsule and preparation method thereof
A technology for norfloxacin and capsules, which is applied to the field of norfloxacin oral capsules and their preparation, can solve the problems of slow dissolution rate and unattainable curative effect, and achieves the effects of stable quality, definite curative effect and simple production process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] An oral capsule of norfloxacin of this embodiment includes norfloxacin, sodium starch glycolate and pharmaceutically acceptable excipients, wherein the mass ratio of norfloxacin to sodium starch glycolate is 23.8:1.
[0038] The contents of the capsule are composed as follows:
[0039]
[0040] Wherein: the particle size of the norfloxacin raw material is D50≤60μm, D90≤180μm; the particle size of the sodium starch glycolate is D50≤30μm, D90≤75μm.
[0041] The preparation method of the aforementioned norfloxacin oral capsule is as follows:
[0042] Sieve norfloxacin into particle size D50≤60μm, D90≤180μm, sodium starch glycolate to filter out particle size D50≤30μm, D90≤75μm, and then separate norfloxacin and sodium starch glycolate in a three-dimensional mixer Mix for 10 minutes, then add the prescribed amount of lactose, silicon dioxide, and magnesium stearate and mix for 5 minutes in a three-dimensional mixer; finally, fill in the 2# capsule shell at 278 mg / capsule to make 10...
Embodiment 2
[0044] An oral capsule of norfloxacin of this embodiment includes norfloxacin, sodium starch glycolate and pharmaceutically acceptable excipients, wherein the mass ratio of norfloxacin to sodium starch glycolate is 13.3:1.
[0045] The contents of the capsule are composed as follows:
[0046]
[0047] Wherein: the particle size of the norfloxacin raw material is D50≤60μm, D90≤180μm; the particle size of the sodium starch glycolate is D50≤30μm, D90≤75μm.
[0048] The preparation method of the aforementioned norfloxacin oral capsule is as follows:
[0049] Sieve norfloxacin into particle size D50≤60μm, D90≤180μm, sodium starch glycolate to filter out particle size D50≤30μm, D90≤75μm, and then separate norfloxacin and sodium starch glycolate in a three-dimensional mixer Mix for 10 minutes, then add the prescribed amount of lactose, silicon dioxide, and magnesium stearate, mix for 5 minutes in a three-dimensional mixer, and finally fill in the 2# capsule shell at 260 mg / capsule to make 10...
Embodiment 3
[0051] An oral capsule of norfloxacin of this embodiment includes norfloxacin, sodium starch glycolate and pharmaceutically acceptable excipients, wherein the mass ratio of norfloxacin to sodium starch glycolate is 16.6:1.
[0052] The contents of the capsule are composed as follows:
[0053]
[0054] Wherein: the particle size of the norfloxacin raw material is D50≤60μm, D90≤180μm; the particle size of the sodium starch glycolate is D50≤30μm, D90≤75μm.
[0055] The preparation method of the aforementioned norfloxacin oral capsule is as follows:
[0056] Sieve norfloxacin into particle size D50≤60μm, D90≤180μm, sodium starch glycolate to filter out particle size D50≤30μm, D90≤75μm, and then separate norfloxacin and sodium starch glycolate in a three-dimensional mixer Mix for 10 minutes, then add the prescribed amount of lactose, silicon dioxide, and magnesium stearate, mix for 5 minutes in a three-dimensional mixer, and finally fill in the 2# capsule shell at 259 mg / capsule to make 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| D90 | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com