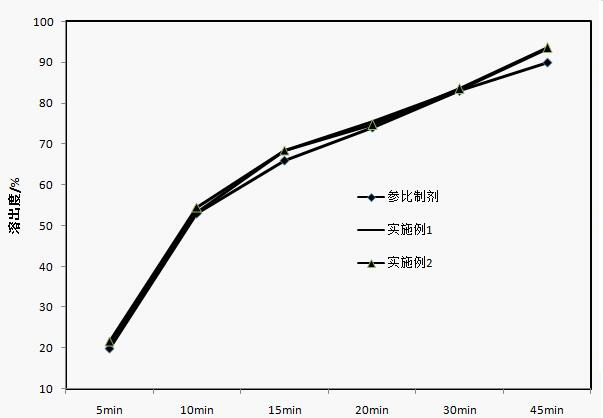
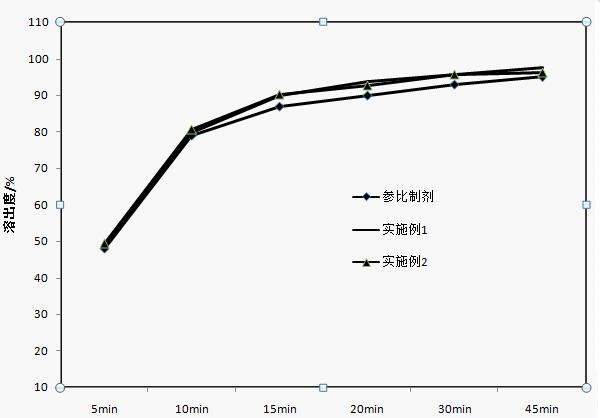
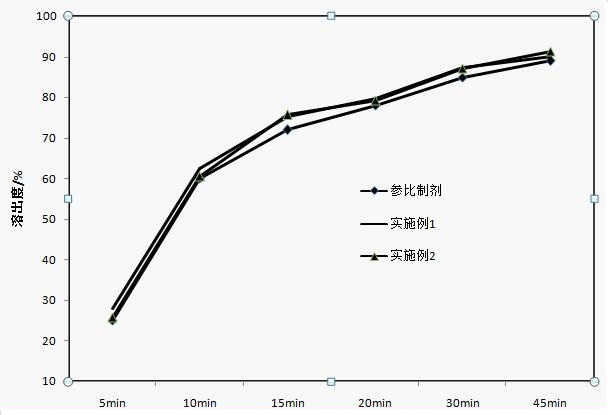
Apixaban tablet composition and preparation method thereof
A technology of apixaban tablets and apixaban, which is applied in the field of medicine and can solve the problems of water solubility not being affected by pH value, slow dissolution rate of apixaban tablets, and low content of active ingredients of the main drug , to achieve the effect of reducing medical costs, determining the curative effect and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Apixaban tablet composition
[0054] Tablet core prescription composition:
[0055]
[0056] Preparation:
[0057] (1) Preparation of raw and auxiliary materials
[0058] ①Preparation of adhesive: prepare 7.2% aqueous solution of povidone K30;
[0059] ②The average particle size of microcrystalline cellulose PH101 is 65 microns; the average particle size of PH102 is 130 microns; the D90 of apixaban is 6.2 microns;
[0060] (2), premixing, wet granulation
[0061] Add apixaban, lactose, microcrystalline cellulose PH101, croscarmellose sodium, and sodium lauryl sulfate into a wet mixing granulator for premixing, and then add the binder under stirring conditions, Wet granulation; parameters of premixing and wet granulation are as follows:
[0062]
[0063] (3) Drying: Dry at 50-55°C to a moisture content of 1.6%;
[0064] (4), granulation: add the dried granules into the granulator for granulation, and the rotating speed is 600rpm; after finishing...
Embodiment 2
[0071] Embodiment 2: Apixaban tablet composition
[0072] Tablet core prescription composition:
[0073]
[0074] Preparation:
[0075] (1) Preparation of raw and auxiliary materials
[0076] ①Preparation of adhesive: prepare 7.2% aqueous solution of povidone K30;
[0077] ②The average particle size of microcrystalline cellulose PH101 is 65 microns; the average particle size of PH102 is 130 microns; the D90 of apixaban is 6.2 microns;
[0078] (2), premixing, wet granulation
[0079] Add apixaban, lactose, microcrystalline cellulose PH101, croscarmellose sodium, and sodium lauryl sulfate into a wet mixing granulator for premixing, and then add the binder under stirring conditions, Wet granulation; parameters of premixing and wet granulation are as follows:
[0080]
[0081] (3) Drying: Dry at 50-55°C to a moisture content of 1.8%;
[0082] (4), granulation: add the dried granules into the granulator for granulation, and the rotating speed is 600rpm; after finishing...
Embodiment 3
[0089] Embodiment 3: Apixaban tablet composition
[0090] Tablet core prescription composition:
[0091]
[0092] Preparation:
[0093] (1) Preparation of raw and auxiliary materials
[0094] ①Preparation of adhesive: prepare an aqueous solution of 8.0% povidone K30;
[0095] ②The average particle size of microcrystalline cellulose PH101 is 65 microns; the average particle size of PH102 is 130 microns; the D90 of apixaban is 6.2 microns;
[0096] (2), premixing, wet granulation:
[0097] Add apixaban, lactose, microcrystalline cellulose PH101, croscarmellose sodium, and sodium lauryl sulfate into a wet mixing granulator for premixing, and then add the binder under stirring conditions, Wet granulation; parameters of premixing and wet granulation are as follows:
[0098]
[0099] (3) Drying: Dry at 50-55°C to a moisture content of 1.4%;
[0100] (4), granulation: add the dried granules into the granulator for granulation, and the rotating speed is 500rpm; after finis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com