Formula and technology for improving strong wet-absorbing performance and dissolving-out behavior of levocarnitine tablets
A technology of L-carnitine tablet and hygroscopicity is applied in the field of improving the strong hygroscopicity and dissolution behavior of L-carnitine tablet, which can solve the problems of cracking of the tablet, easy moisture absorption of L-carnitine, sticking and punching of materials, etc. Consistent quality, solve the effect of easy moisture absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
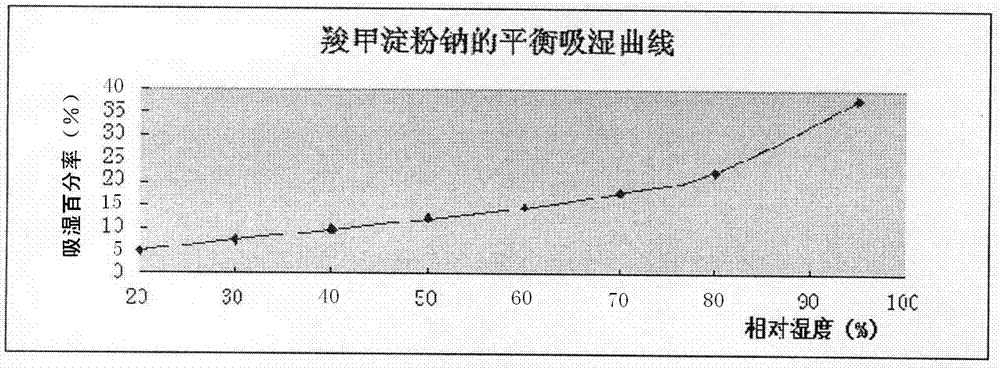
[0017] According to the prescription in Table 1, 6.6kg of levocarnitine and 3.84kg of mannitol were mixed evenly in a high-efficiency wet mixing granulator (HLSG20B type, Beijing Aviation Manufacturing Engineering Research Institute of Bank of China Industry), and then added sodium starch glycolate in order 0.6kg, povidone K900.72kg, fumed silicon dioxide 0.18kg, and magnesium stearate 0.06kg were uniformly mixed, and the mixture was directly compressed into powder in a high-speed rotary tablet press (ZP35D type, Liaocheng Wanhe Industrial Manufacturing Co., Ltd.) (RH% control in the GMP workshop is 40%-60%), tablet diameter 13mm, shallow concave, thickness 5.30-5.60mm, plain tablet weight 600mg.
[0018] Table 1. Composition of generic L-carnitine tablet-330mg
[0019]
example 3
[0025] Under different relative humidity conditions at 25°C, the equilibrium moisture absorption curve of levocarnitine (pharmaceutical grade, Northeast Pharmaceutical Group Co., Ltd.) was measured with a moisture absorption analyzer (AQU ALB, Decagon Devices, Inc.), and the results are shown in figure 1 ..
example 4
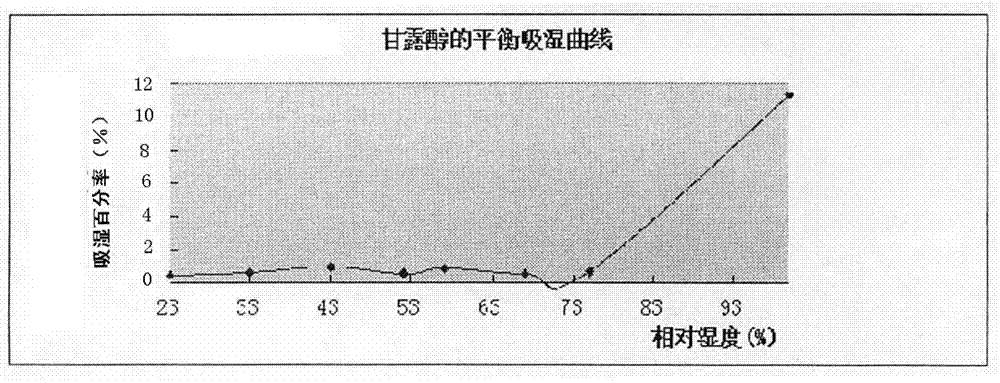
[0027] Under different relative humidity conditions at 25°C, the equilibrium moisture absorption curve of mannitol (pharmaceutical grade, Hebei Huaxu Pharmaceutical Co., Ltd.) was measured with a moisture absorption analyzer (AQUA LAB, Decagon Devices, Inc.), and the results are shown in figure 2 ..
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com