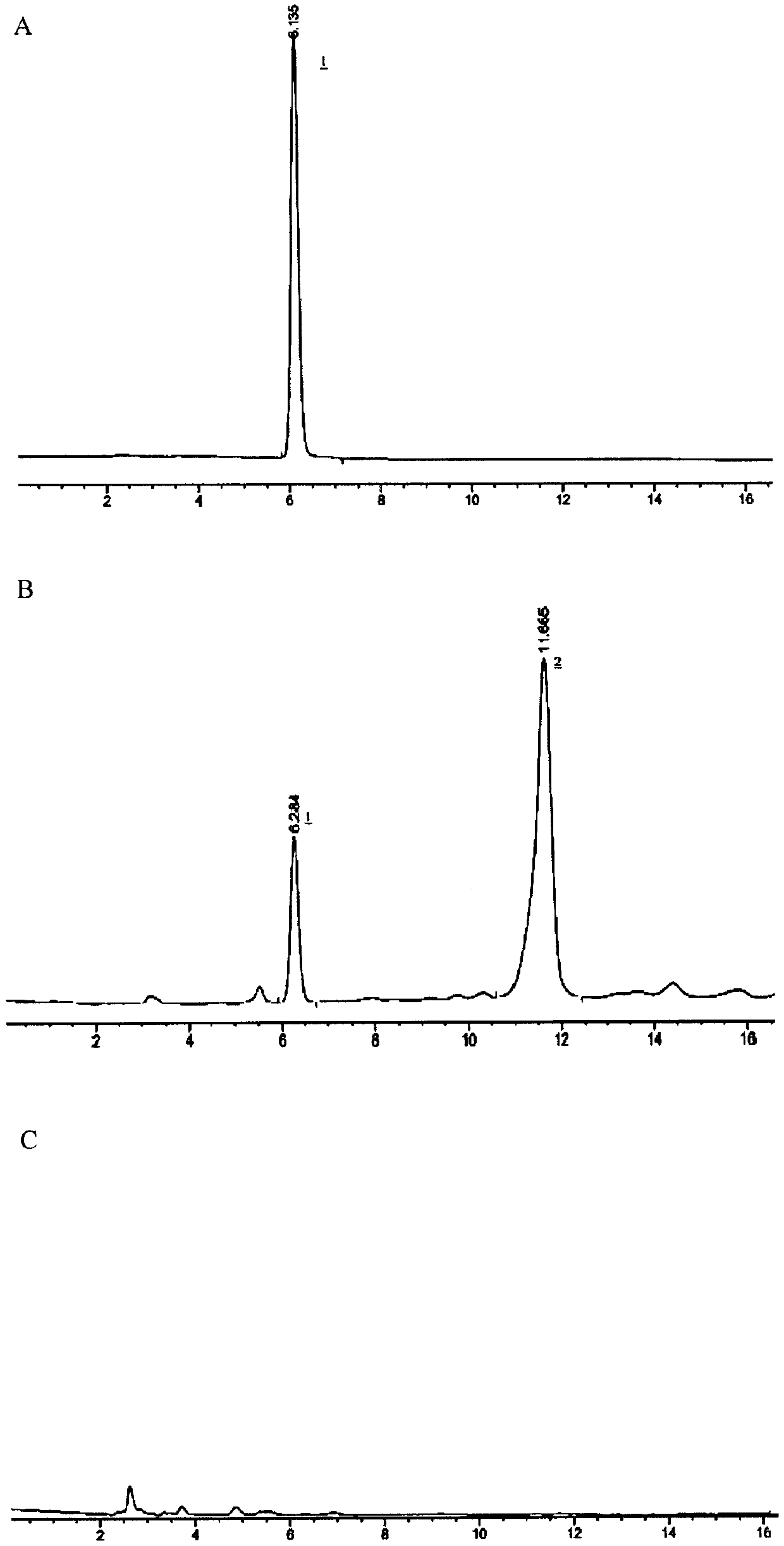
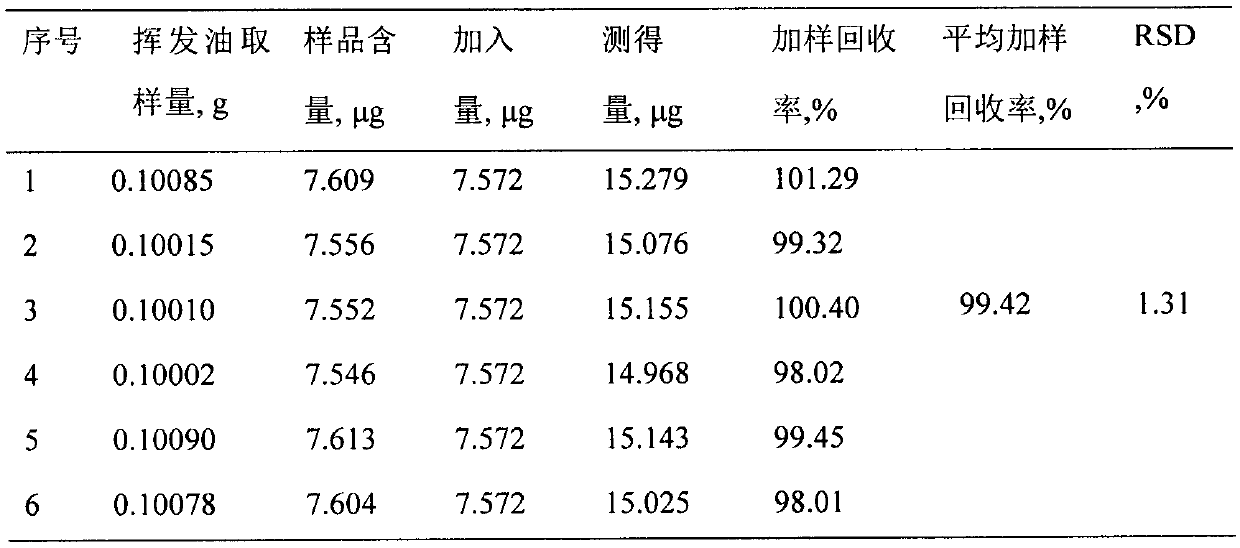
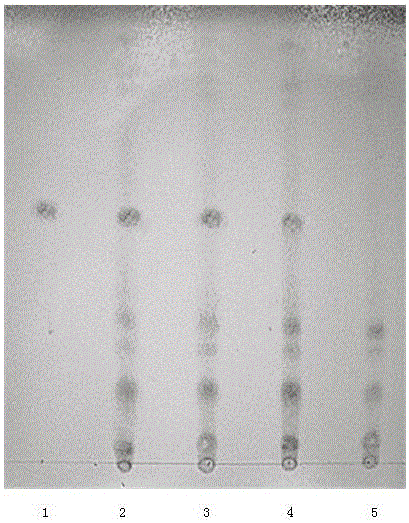
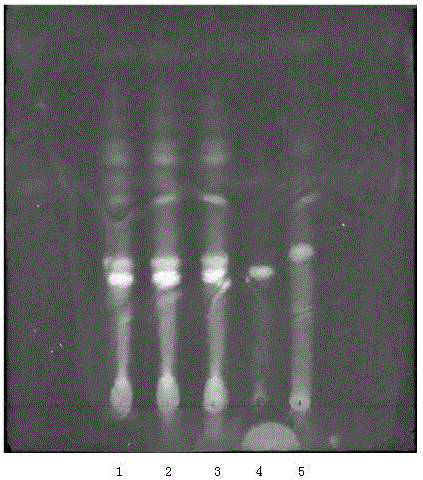
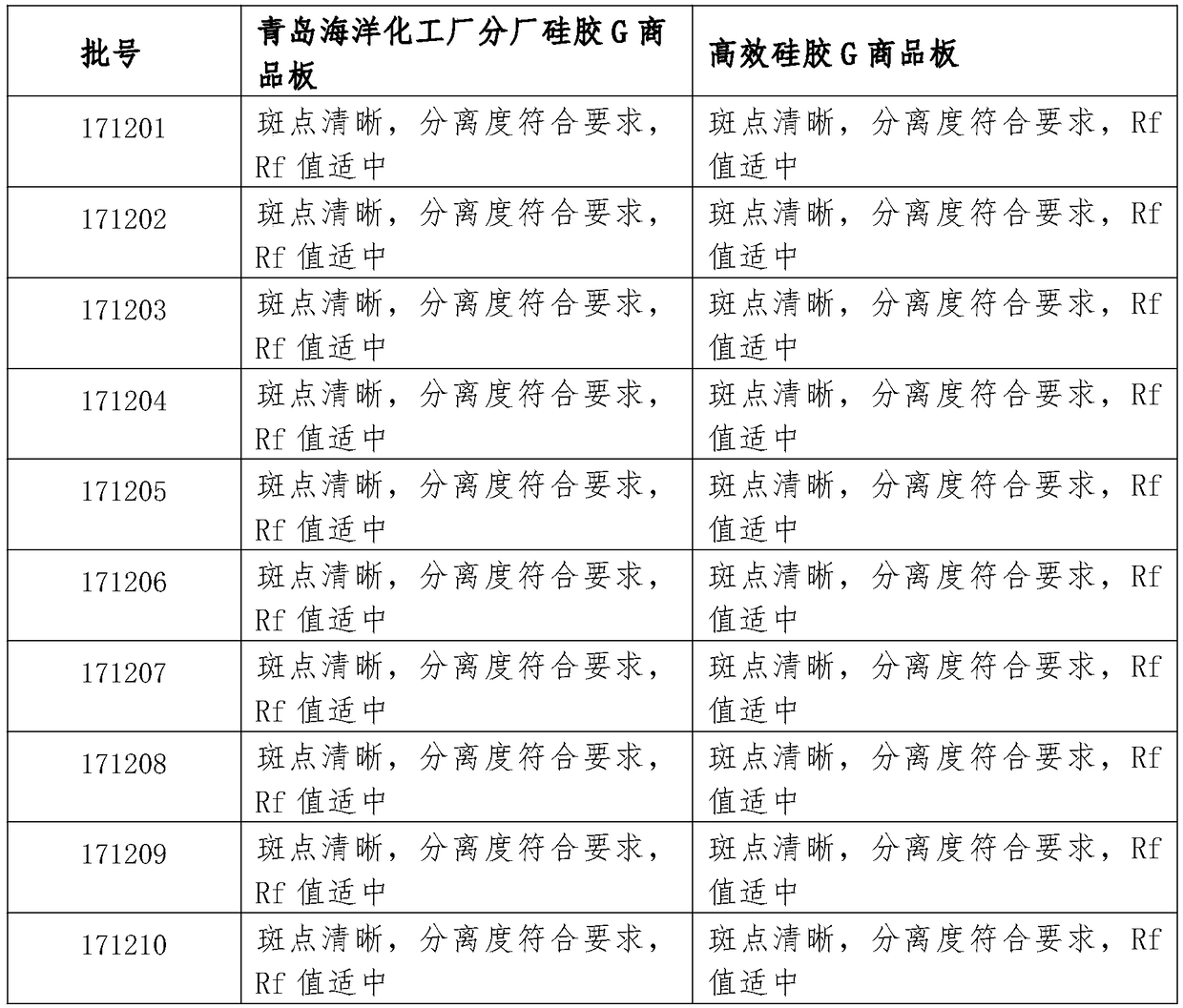
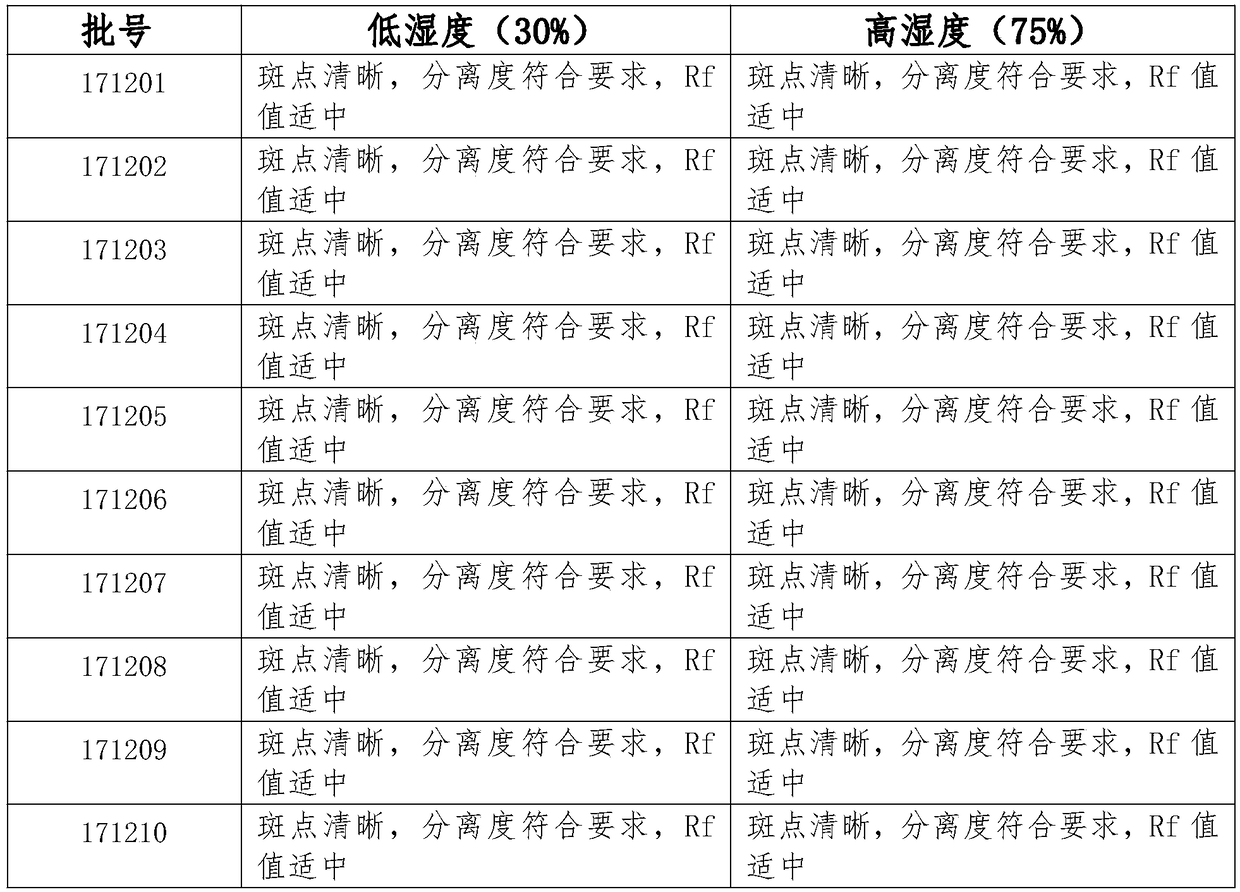
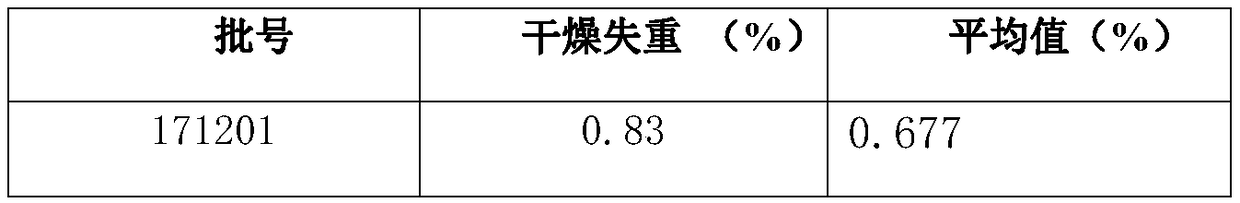
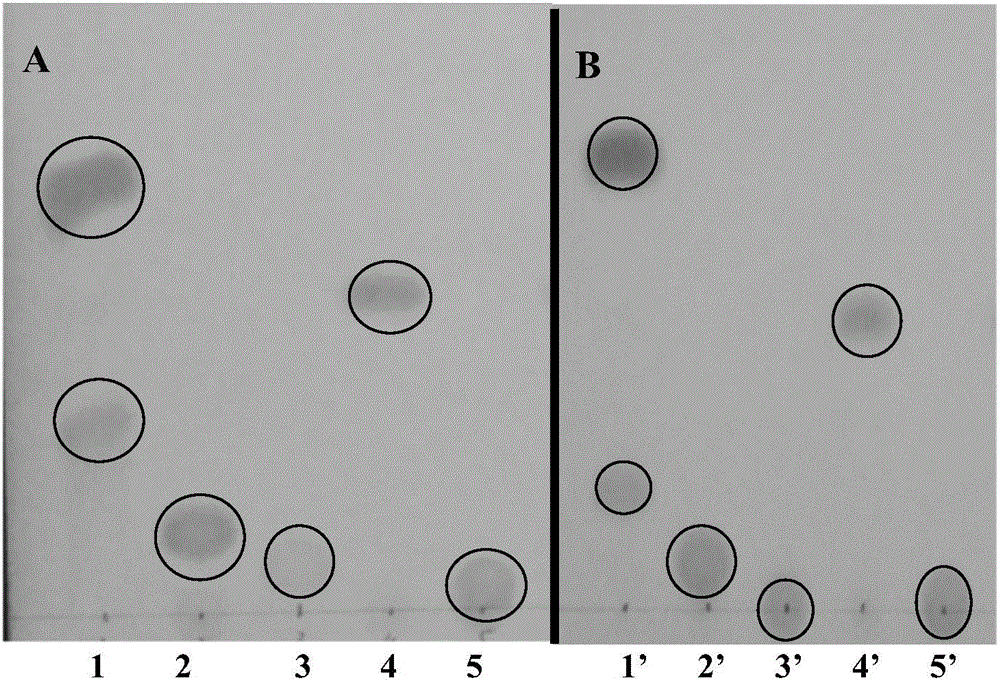
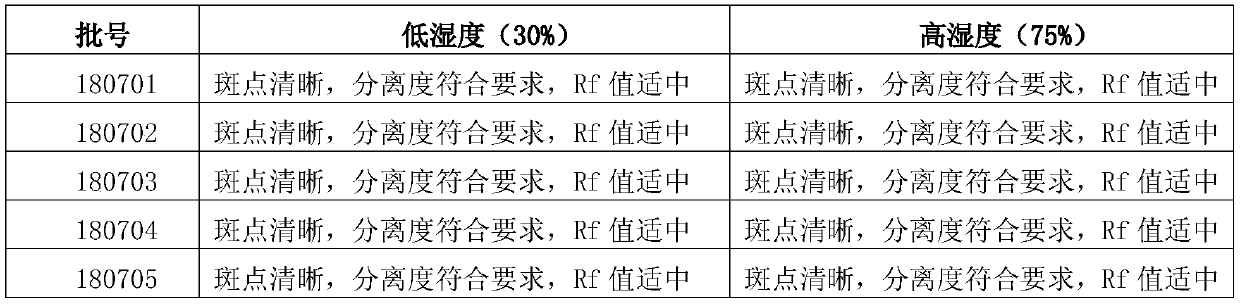
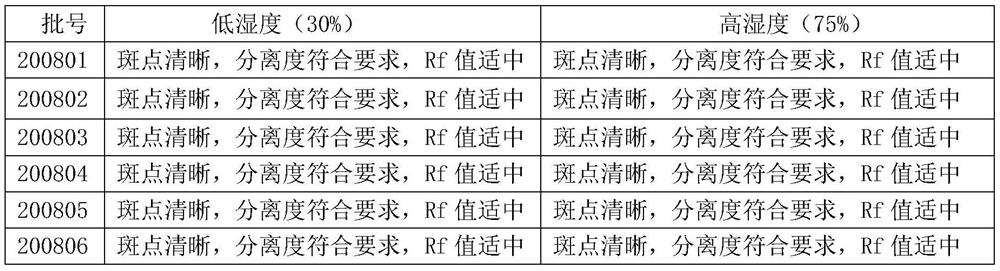
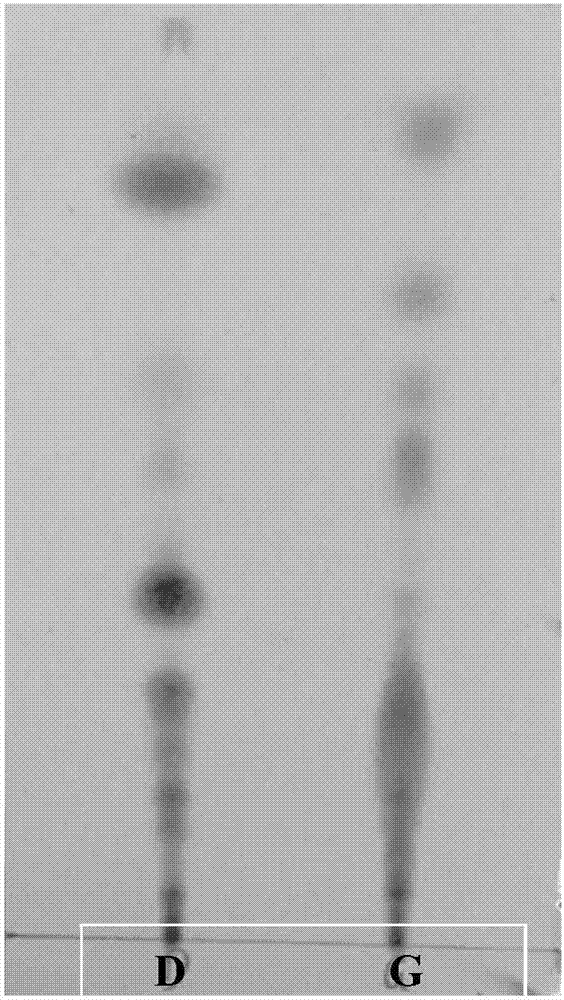
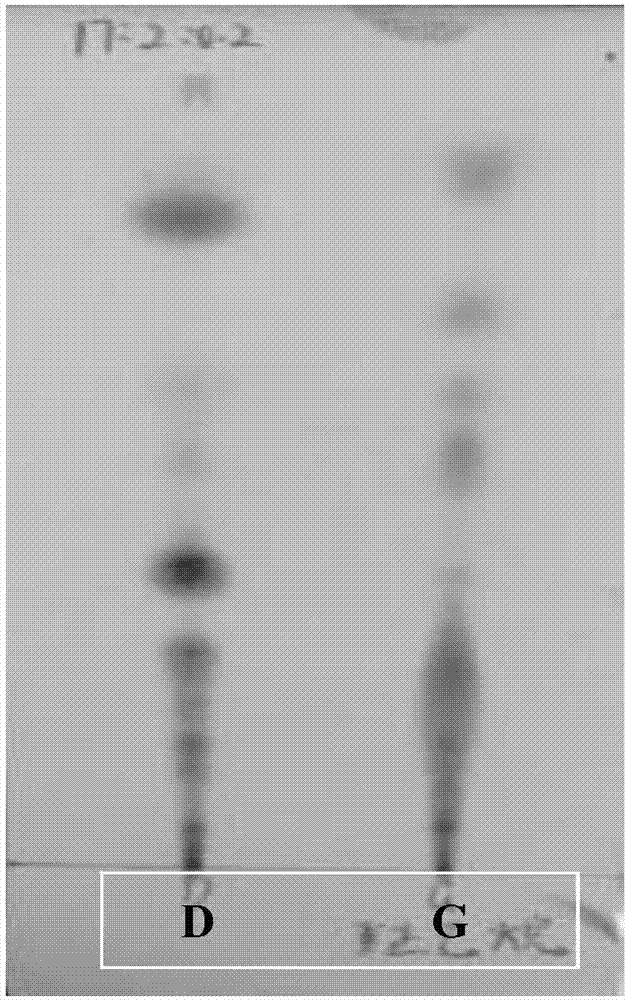
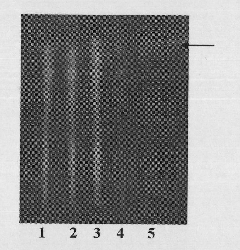
Patents
Literature
69results about How to "Clear spots" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
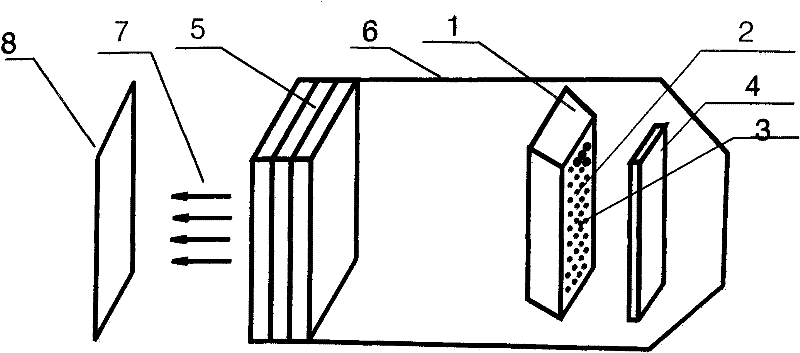
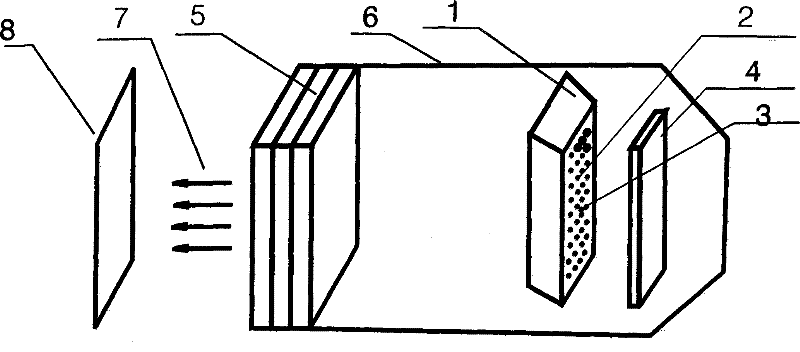
LED illumination light source
InactiveCN102691892ASolve application problemsSolve the glare problem of normal drivingPoint-like light sourceElectric lightingLight beamTemperature Compensator
The invention relates to an LED illumination light source comprising a LED light-emitting array, a colour temperature compensator, an optical light-equalizing mirror, a light gathering device, a light gathering cover, a light after gathering and a target light spot. The LED illumination light source is an LED novel light source that allows colour temperature to be adjustable and can be organically combined with the light gathering device. The a light beam that has been optically processed forms a uniform and clear rectangular light spot with a fixed size at a predetermined target place in front of the light gathering device. The size, the light uniformity and the sharpness of the rectangular light spot can be adjusted through adjusting a focal length among the light gathering device, the optical light-equalizing mirror and the LED light-emitting array.
Owner:李姝江
Chinese medicine preparation Taohong pill for regulating menstruation and relieving pain
InactiveCN102240348ASimple preparation processStrict quality control methodsAntipyreticAnalgesicsAngelica Sinensis RootSemen
The invention relates to a Chinese medicine preparation, concretely relates to a Chinese medicine preparation Taohong pill for regulating menstruation and relieving pain, and belongs to the technical field of the Chinese medicine preparation. According to the invention, semen persicae and safflower are taken as principal drugs which are used for promoting blood circulation and removing blood stasis, as well as regulating menstruation and relieving pain, and the principal drugs are supplied by angelica sinensis, hemlock parsley, Radix Paeoniae rubra, Radix Rehmanniae Preparat for nourishing yin and supplementing blood, nourishing blood for regulating menstruation which are capable of assisting the principal drugs to activate blood and remove stasis; and accompanied by cattail pollen, Trogopterus Dung for promoting blood circulation and getting rid of the stale and bringing forth the fresh; and Rhizoma Corydalis is supplied for invigorating the blood circulation and promoting the circulation of qi to relief pain; all traditional Chinese herbs of the prescription are used together for promoting the circulation of qi and blood circulation, dredging collaterals and relieving pain.
Owner:杜文玲
Compound black-bone chicken soft capsule, its preparing method and quality control method
InactiveCN1814126AF value is suitableClear spotsComponent separationMammal material medical ingredientsLiver and kidneyCodonopsis
The invention relates to a Chinese medicine-compound black-bone chicken soft capsule having functions of tonifying Qi and blood and benefiting liver and kidney and the preparing and quality control methods thereof. It has many advantages of stability, convenient to take and carry, no sugar, no bad smell, etc, and the quality control method comprises identifying White Paeony Root, cortex moutan radicis, Milkvetch Root, Radix Codonopsis, Chinese Angelica Root and Rhizoma Chuanxiong in the soft capsule, making content determination on the active substance of White Paeony Root and cortex moutan radicis-paeoniflorin in the soft capsule and controlling total nitrogen content of the soft capsule.
Owner:毛晓敏
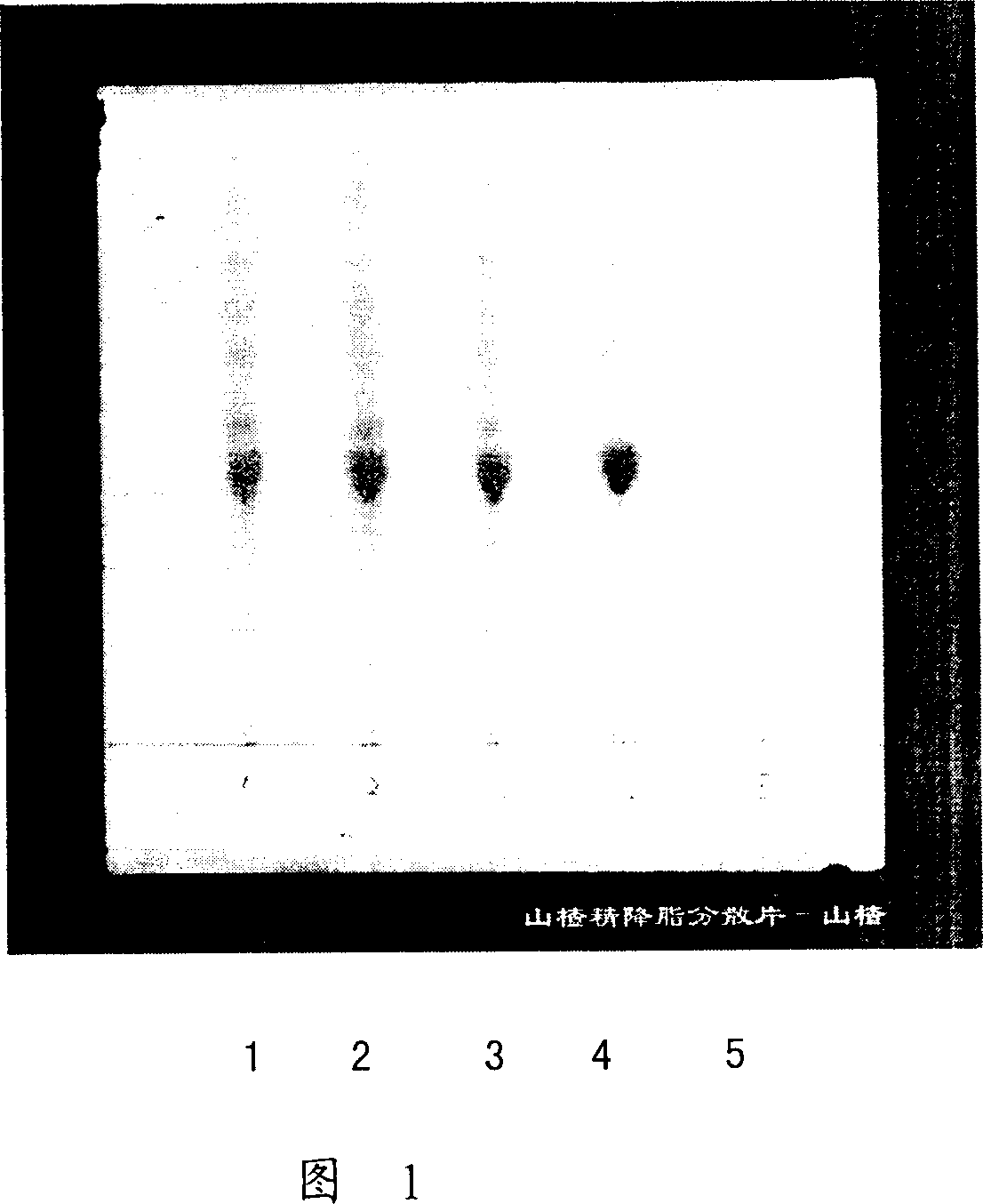
Method for identifying hawthorn of hawthorn extract lipid-lowering dispersion tablet
InactiveCN101008634ASimple and fast operationEasy to separateComponent separationMaterial analysis by observing effect on chemical indicatorEngineeringLipid lowering
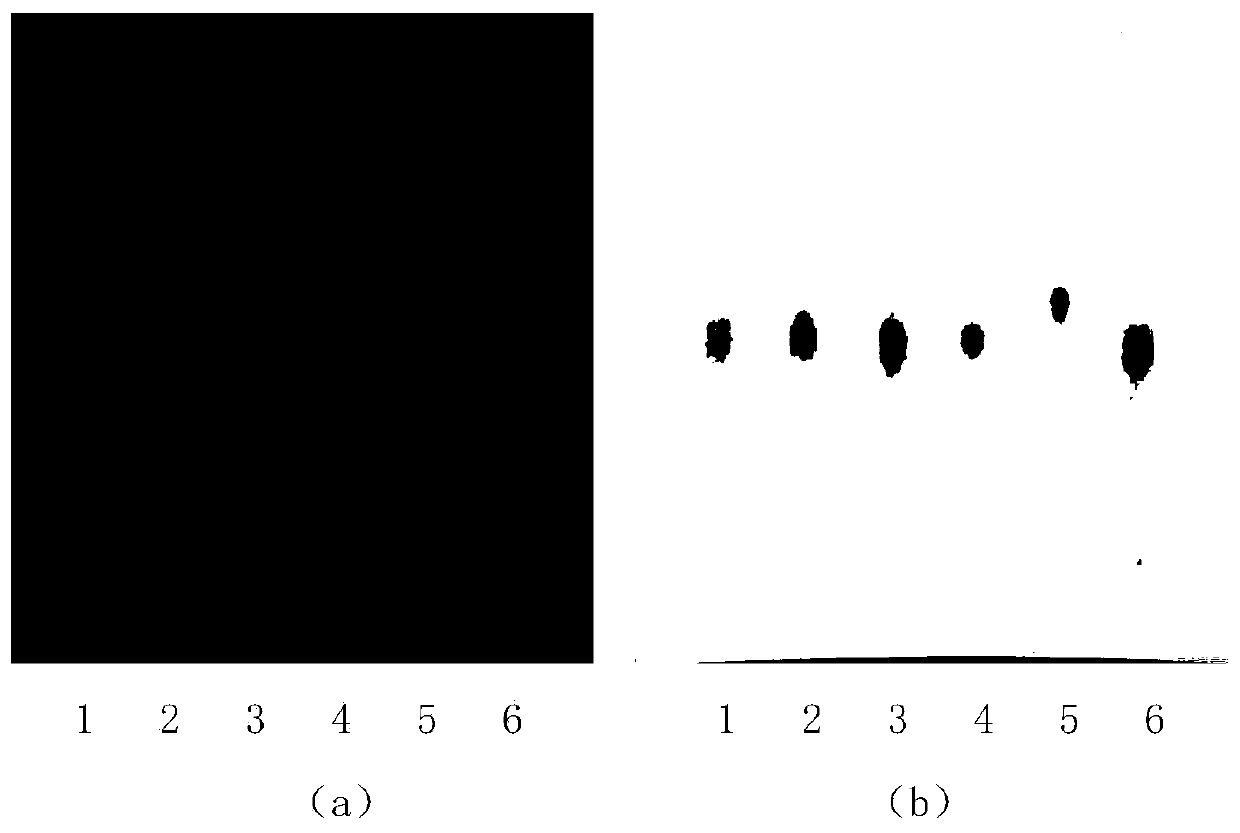
This invention relates to haw antihyperglycemic slice identification method, which adopts film chromatography spectrum as haw antihyperglycemic slice qualitative identification of simple operations and good isolation and high accuracy and clear spot through negative lighting without interference with operating property belonging to haw antihyperglycemic slice quality standard.
Owner:NINGXIA QIYUAN PHARMA
Thin-layer chromatographic developer for detecting residual amount of tripolycyanamide and application of thin-layer chromatographic developer
InactiveCN102175812AClear spotsHigh detection sensitivityComponent separationThin layer chromatographicUltraviolet lights
The invention discloses a thin-layer chromatographic developer for detecting residual amount of tripolycyanamide and application of the thin-layer chromatographic developer. The developer contains 0.1 to 10 weight percent of p-dimethylaminobenzaldehyde and 0.1 to 2 weight percent of ethanol solution of inorganic acid such as hydrochloric acid or sulfuric acid respectively. During detection, a tested sample solution which contains tripolycyanamide and is sufficiently dissolved in a tripolycyanamide organic good solvent with the boiling point of less than or equal to 100 DEG C is applied to a silica gel chromatoplate and developed by the developer in a conventional way and then the developer is volatilized; after a sample is developed by the developer, development spots on the thin-layer chromatoplate are observed under an ultraviolet light or a natural light; and whether the tripolycyanamide content of the tested sample surpasses the prescribed limit is computed according to the amount of the applied sample. By the method, the spots of tripolycyanamide are clear; the detecting sensitivity is high; and the minimum detection value of tripolycyanamide can be 0.5mg.kg<-1>. The method is applicable for detecting the tripolycyanamide content of the food for people and / or animals, including dairy products.
Owner:SICHUAN UNIV
Detection method of traditional Chinese medicinal composition for treating waist and knee pains and sciatica
ActiveCN106918674AIncrease quantitative detectionControl internal qualityComponent separationSciaticaBoswellia
The invention provides a detection method of a traditional Chinese medicinal composition for treating waist and knee pains and sciatica. Raw materials of the traditional Chinese medicinal composition comprise Rhizoma Cibotii, Fructus Rosae Laevigatae, Caulis Spatholobi, Philippine flemingia root, Kadsura coccinea, Millettia specisoa Champ, Ligustri Lucidi Fructus, Chinese Taxillus Twig, semen cuscutae, Rhizoma Corydalis, Zanthoxylum nitidum, Boswellia carteri and myrrh. The detection method comprises the following steps: detecting the content of (2'S)-Kadsura longepedunculata lignin J in the Kadsura coccinea through high performance liquid chromatography; and detecting the content of specnuezhenide in the Ligustri Lucidi Fructus through the high performance liquid chromatography, and detecting the Kadsura coccinea, the Rhizoma Cibotii and the Chinese Taxillus Twig through thin layer chromatography. The invention also concretely discloses conditions and concrete operating steps of all above detection technologies. The detection method allows five raw materials to be qualitatively and quantitatively detected, and the quantity of the detected raw materials is 1 / 3 of the total quantity of the raw materials, so the quality of medicines is effectively guaranteed, and medical demands are met.
Owner:GUANGZHOU CHEN LI JI PHARMA FACTORY
Quality detecting method of medicine composition for treating obsessed feeling
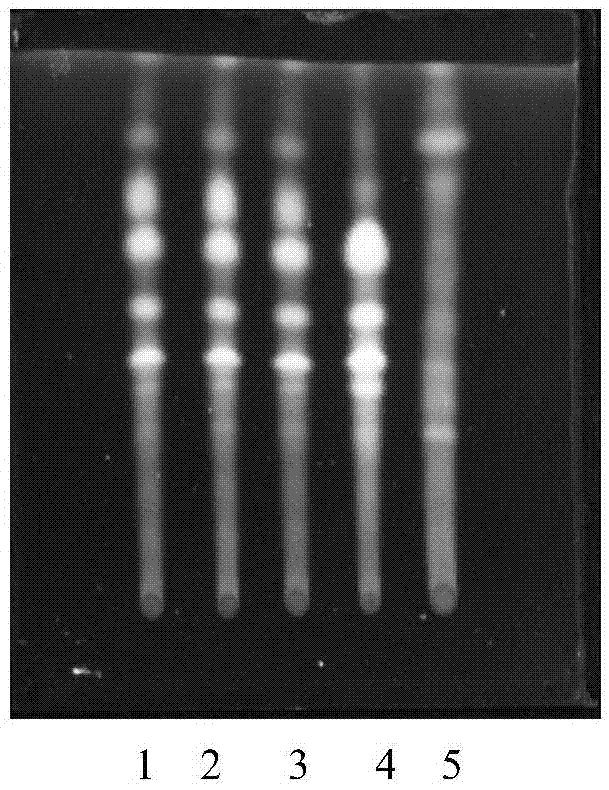
ActiveCN109406651AQuality improvementAdd TLC DiscriminationComponent separationAdditive ingredientHypnotic Effects
The invention relates to a quality detecting method of a medicine composition for treating obsessed feeling. The medicine composition is prepared from semen ziziphi spinosae, radix bupleuri, white paeony roots, flos albizziae, cortex albiziae, bombyx batryticatus, cicada slough and junci medulla. The quality detecting method comprises the steps of distinguishing the active ingredients of radix bupleuri, flos albizziae, cortex albiziae, bombyx batryticatus and junci medulla, and determining the content of paeoniflorin in white paeony root, jujuboside A in semen ziziphi spinosae and spinosin. The jujuboside in semen ziziphi spinosae and flavonoid ingredient spinosin have important sedative and hypnotic effects; a basis is provided for evaluating the quality of semen ziziphi spinosae. The medicine quality is comprehensively and effectively controlled; an efficient, strict and reliable quality detecting method is made for ensuring the medicine quality; the accuracy is high; the stability is high; the sample treatment time is saved; the method can be effectively used for the quality detection on the medicine composition for treating obsessed feeling.
Owner:贵州大隆药业有限责任公司
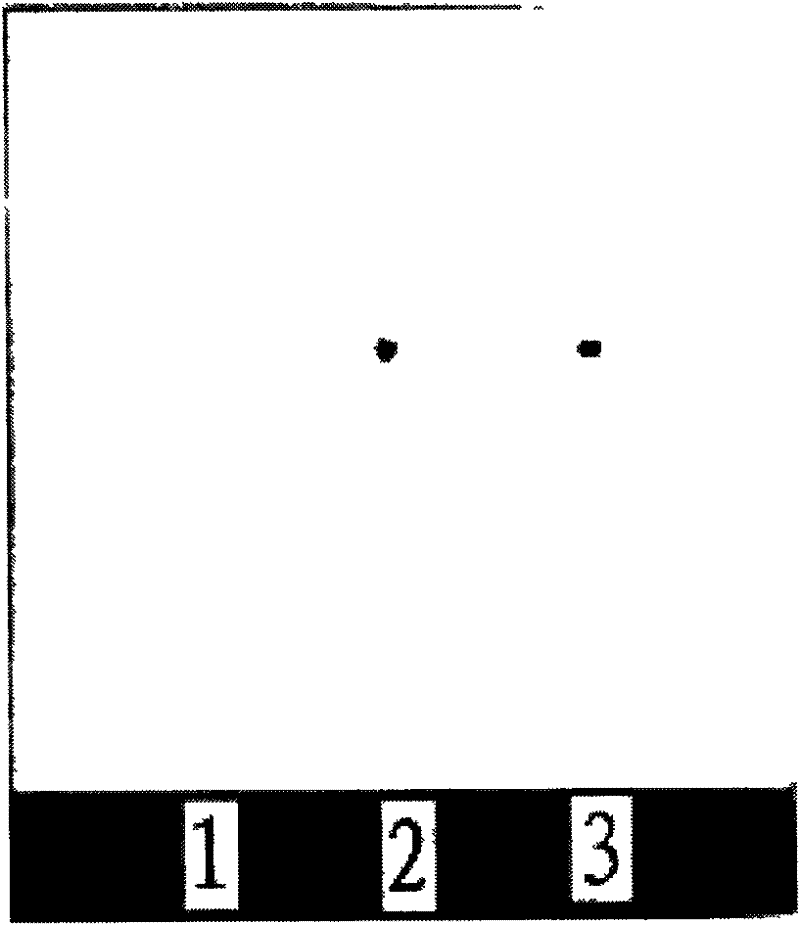
Method for identifying codonopsis pilosula and preparation containing codonopsis pilosula
InactiveCN101084972AClear spotsMaterials are readily availableComponent separationPlant ingredientsMedicinal herbsMedicinal herb
The invention provides a medicinal herb of radix codonopsis and a method for discriminating preparation containing radix codonopsitis. The method comprises the following steps in sequence: preparation of testing solution for assay: a preparing medicinal herb of radix codonopsitis, extract of radix codonopsitis, or preparation containing radix codonopsitis into aqueous solution, loading on macroporous adsorbent resin column, eluting with 30-70& lower alcohol and 75-100% lower alcohol in sequence, collecting eluant of 75-100% lower alcohol, concentrating to dry, and dissolving in lower alcohol as testing solution for assay; b preparing contrast medicinal herb of radix codonopsitis into contrast solution according to the preparation method of testing solution for assay; c sucking testing solution for assay and contrast solution, dotting on the same silica gel g plate, developing with n-butanol-glacial acetic acid-water of ratio of 1-10:1:0.2-7 as developer, taking out, air-drying, and detecting with ultraviolet lamp; wherein steps a and b has no precedence order. The inventive method has simple operation and low cost.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Quality control method of xiaojiean preparation
ActiveCN102198210AAccurate identificationReliable identificationComponent separationAntineoplastic agentsMotherwortMedicine
The invention relates to a quality control method of a medicine preparation, and especially relates to thin layer chromatogram authentication and content determination of smilax glabra in a xiaojiean preparation. The method is a thin layer chromatogram authentication and / or content determination method formulated by using a special component in the smilax glabra of the xiaojiean preparation as an index component, wherein the special component is astilbin, and the xiaojiean preparation is a traditional Chinese medicine compound preparation prepared from 1100 parts of leatherleaf mahonia, 750 parts of thin evodia, 750 parts of motherwort, 750 parts of spatholobus stem, 900 parts of smilax glabra and 750 parts of fructus forsythiae. The thin layer chromatogram authentication method with a strong specialization can authenticate smilax glabra accurately and reliably, and a generally employed HPLC can determine the astilbin content in the xiaojiean preparation, so as to produce valuable significance for monitoring and controlling medicinal material purchase, preparation production process and preparation quality in market, and ensuring product safety, effectiveness and quality stabilization. According to the method, problem of confused smilax glabra basic material provided in the market can be solved effectively to ensure that xiaojiean preparation meets the national medicine standards strictly.
Owner:云南神威施普瑞药业有限公司
Detection method of ginkgo dipyridamole injection preparation
ActiveCN111983077ASimple processing methodLow toxicityComponent separationGinkgo Biloba Leaf ExtractClinical efficacy
The invention provides a detection method of a ginkgo dipyridamole injection preparation. The ginkgo dipyridamole injection preparation is prepared from a ginkgo leaf extract and dipyridamole, and thedetection method comprises the items of character, identification, inspection, fingerprint spectrum and content determination. Compared with the prior art, a thin-layer chromatography identificationmethod of the ginkgo biloba extract, limited quantity inspection of 5-hydroxymethylfurfural, total ginkgolic acid and the like, inspection of injection related substances, fingerprint detection and the method for determining the content of rutin, kaempferol-3-O-rutinoside, narcissoside and terpene lactones are additionally arranged, the method for determining the content of the total flavonol glycosides is optimized, the requirement for product quality detection is improved, and the method is more suitable for quality control of drugs in the future and is more beneficial to guaranteeing the drug quality and clinical efficacy.
Owner:GUIZHOU YIBAI PHARMA CO LTD
Focusing lamp lens set and corresponding lighting system
PendingCN108826229AOvercome the problem of layeringEnsure light utilizationMountingsCondensersRefractive indexLighting system
The invention relates to a focusing lamp lens set. The focusing lamp lens set comprises a first lens and a lens assembly, wherein the space between the first lens and a light source is fixed, the first lens emits all lights of the light source, and the lens assembly is located on the light emitting side of the first lens; the lens assembly comprises one or more concave lenses and one or more convex lenses, wherein the convex lenses are located between the first lens and the concave lenses; the convex lenses and the concave lenses have the same radius and the same refractive index, the convex faces of the convex lenses are opposite to the concave faces of the concave lenses, and the space between the convex lenses and the concave lenses can be adjusted so that different emitting angles canbe obtained. By the adoption of the focusing lamp lens set, the light efficiency is improved, and the irradiation effect is good.
Owner:SELF ELECTRONICS CO LTD
Kidney-yang-warming medicine composition, and preparation thereof and preparation method thereof
ActiveCN102895387APlay the role of warming and tonifying kidney yangHas the effect of treating kidney-yang deficiency syndromeComponent separationDigestive systemActive componentYang deficiency
The invention relates to a kidney-yang-warming medicine composition, and a preparation thereof and a preparation method thereof. An active component of the composition is prepared from, by weight: 312-440 parts of silky ant, 24-80 parts of hairy antler pilose antler, 16-20 parts of Chinese caterpillar fungus, 48-110 parts of kirilow rhodiola root and rhizome, 168-260 parts of Mongolian milkvetch root, 112-190 parts of longan pulp, 312-440 parts of tussah silkworm chrysalis, 64-130 parts of submature bitter orange, and 112-190 parts of common jujube. As a result of pharmaceutical effect experiments, the medicine composition provided by the invention has a function for treating kidney-yang deficiency syndrome. The effect of the composition is better than medicine compositions not provided by the invention.
Owner:吉林省银诺克药业有限公司
Tibetan medicine pill for influenza as well as quality criteria and inspection method of preparation thereof
ActiveCN101502628AQuality improvementReproducibleComponent separationInanimate material medical ingredientsHypecoum erectumThin layer chromatogram
The invention discloses a flu pill of Tibetan medicine and a quality control method of a preparation thereof; wherein the preparation is various preparation formulations which are made by adding conventional excipients according to the conventional technique and the formulation of the flu pill in fascicule of Tibetan medicine of Standards of Medicine of the Ministry of Public Health. The quality control method comprises one or more of identification methods. The invention carries out thin layer chromatogram identification study on hypecoum erectum and bhizoma acori calami in the formulation, establishes the quality control method with reliability, accuracy and strong specificity, can effectively control the quality of the flu pill and enable the flu pill to achieve stable, controllable, high-effective and safe quality.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
Thin-layer identification method for vitality-preserving soup and similar formula extracts and preparations thereof
ActiveCN112697949AAvoid separate sample preparationEasy to separateComponent separationPreparing sample for investigationBiotechnologyAcetic acid
The invention relates to a thin-layer identification method for vitality-preserving soup and similar formula extracts and preparations thereof which can be used for identifying main medicines such as ginseng, astragalus membranaceus and liquorice in the vitality-preserving soup and the similar formula extracts and preparations thereof at the same time, avoids independent sample preparation, plate application, development, color development and inspection of three medicines, and the method is simple to operate, short in detection period and high in inspection efficiency. A thin-layer chromatogram obtained by the method is good in separation degree and clear in spot color development, and the use of irritant reagents such as ammonia water, trichloromethane and glacial acetic acid is avoided.
Owner:浙江金城阜通制药有限公司
TLC (Thin layer chromatography) rapid identification method for cacumen biotae
The invention relates to a TLC (Thin layer chromatography) rapid identification method for cacumen biotae. The method comprises the following steps: taking a to-be-detected sample, adding water, hydrochloric acid and ethyl acetate, heating, refluxing and hydrolyzing in a water bath, filtering while hot, separating the filtrate, washing, evaporating to dryness, adding methanol into the residues for dissolving, and taking the obtained solution as a test solution; and adopting thin layer chromatography, and viewing under an ultraviolet lamp. The method has the advantages of high efficiency, convenience, good reproducibility, clear chromatography, good separation of spots, and the like.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Quality control method of podocarpus macrophyllus fruit medicinal material
The invention discloses a quality control method of a podocarpus macrophyllus fruit medicinal material. The quality control method is characterized in that a chemical component ligustrazine in the medicinal material is taken as a quality control index, a method for identifying ligustrazine in the medicinal material by means of thin-layer chromatography and a method for determining the content of ligustrazine in the medicinal material by means of high performance liquid chromatography are established. The result of the established thin-layer identification method shows that spots are clear, andinterference of other components does not exist; the high performance liquid chromatography target peak has high resolution, good peak shape, high specificity, good reproducibility, high precision and universal applicability and is free of interference of other impurity peaks. The quality control method can scientifically and effectively control the quality of the podocarpus macrophyllus fruit medicinal material.
Owner:GUANGXI MEDICAL UNIVERSITY
Naozhenning thin layer chromatography identification method
InactiveCN107179380AEasy to prepareThe preparation method is simple and environmentally friendlyComponent separationPreparing sample for investigationTest articleQuality control
The invention discloses a TLC identification method for Nazhenning, which belongs to the technical field of quality control of traditional Chinese medicine extracts and preparations. The invention provides the identification of tangerine peel in Naozhenning. The preparation method of the test product is simple, fast, accurate and environmentally friendly. The crude drug contained in it can be quantified, the separation degree is high, the sampling volume is small, the sampling volume is controllable, the sensitivity is high, and the interference is less. The invention provides the identification of Cortex Moutan in Naozhenning, using methanol ultrasonic extraction, water-saturated n-butanol extraction, neutral alumina mixing sample, can obtain the test sample, the sample amount is small, save time, and reduce environmental pollution Less, faster and easier to operate. The method provided by the invention has strong specificity, good reproducibility and durability, and can be used for the identification of the corresponding medicinal flavors in the extracts and preparations of Zhenzhengning.
Owner:SHANXI ZHENDONG ANTE BIOPHARMACEUTICAL CO LTD
Quality detection method for drug combination for treating cough, asthma and thoracic fullness
ActiveCN109374815AShort heating timeShorten drying timeComponent separationToxicantChemical compound
The invention relates to a quality detection method for drug combination for treating cough, asthma and thoracic fullness. The drug combination is prepared from pinecones, cotton roots and folium eriobotryae. The quality detection method comprises the following steps: identifying active ingredients, namely pinecones, cotton roots and folium eriobotryae, and then determining residual quantity of free gossypol in cotton roots. Free gossypol is a polyphenol hydroxyl dinaphthalaldehyde compound, is a toxicant and is capable of resulting in human swelling and bleeding, nervous breakdown, inappetence, weight reduction and influence on fertility. According to the invention, the identification method is optimized on the basis of an original quality standard, and meanwhile, the identification for folium eriobotryae and the determination for the residual quantity of free gossypol in cotton roots are added, so that the quality monitoring for the drug combination for treating cough, asthma and thoracic fullness is greatly promoted. The quality detection method has high accuracy and high stability, can be used for effectively detecting the quality of the drug combination for treating cough, asthma and thoracic fullness and is capable of perfecting the quality standard.
Owner:贵州大隆药业有限责任公司
Method for detecting N-acyl-homoserine lactone quorum sensing signal molecules in sample
ActiveCN105974044AGood repeatabilityHigh resolutionComponent separationSignalling moleculesColor reaction
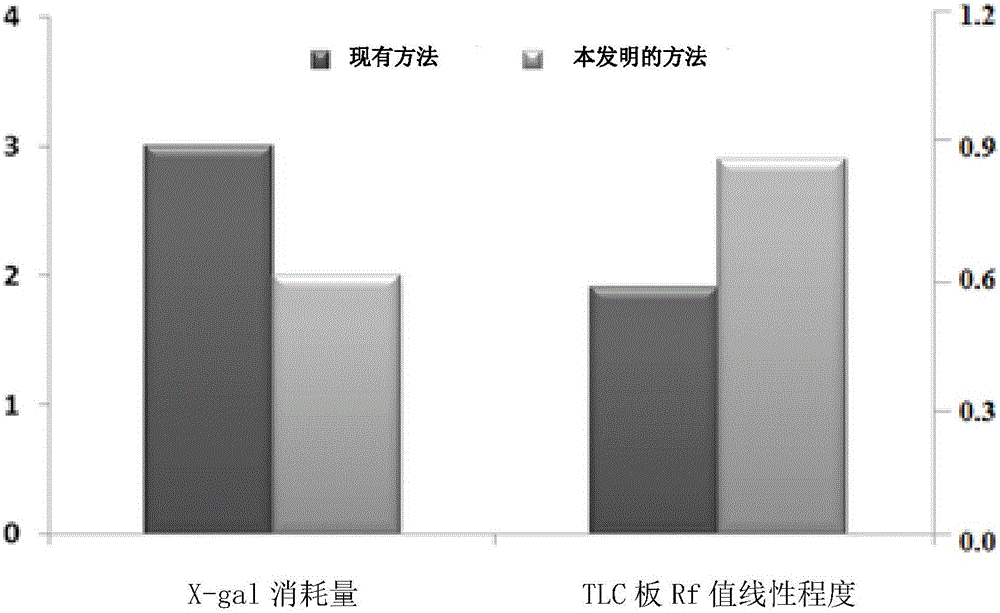
The invention provides a method for detecting N-acyl-homoserine lactone quorum sensing signal molecules in a sample. The method comprises the following steps: (1) placing a pre-prepared semi-solid LB culture medium film on the upper surface of a TLC thin layer chromatoplate, wherein the semi-solid LB culture medium film contains report strains, and the sample is pre-outspreaded on the thin layer chromatoplate; (2) subjecting the film to a chromogenic reaction by utilizing X-Gal; and (3), confirming whether the sample contains the N-acyl-homoserine lactone quorum sensing signal molecules or not based on the results of the chromogenic reaction. The detection method provided by the embodiment of the invention has the advantages of obviously improved repeatability of experimental results, non-diffusion of chromogenic spots, clear color development, high resolution, low amount of X-gal, and high fidelity of detection results.
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
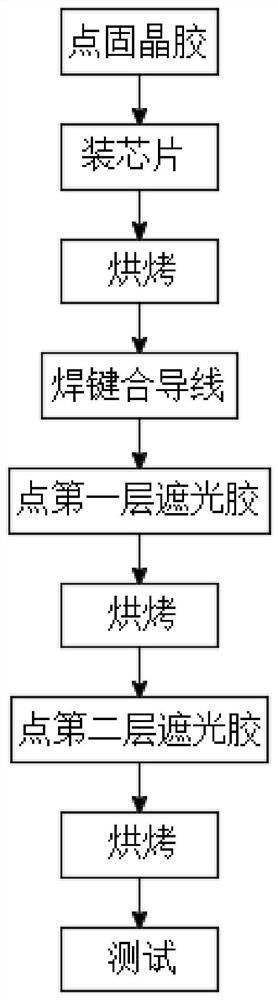
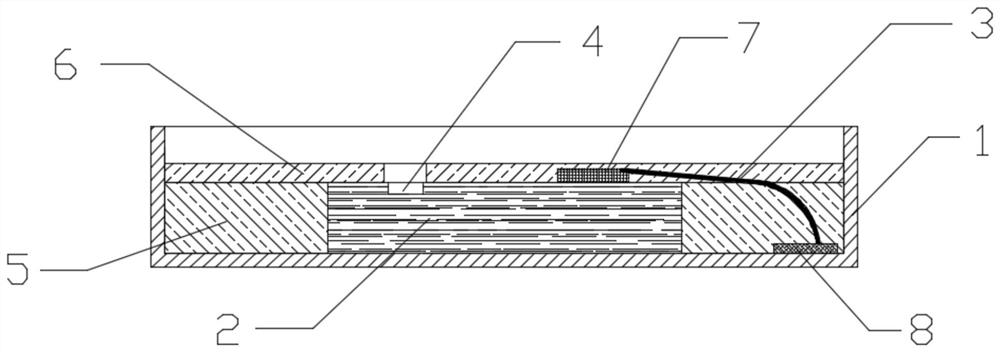
RCLED lamp bead packaging process
ActiveCN113284988AEliminates reflective effectsImprove the effect of emitting light spotsElectric circuit arrangementsCoatingsLight spotEngineering
The invention discloses an RCLED lamp bead packaging process, which comprises the following steps of dispensing solid crystal glue, mounting a chip, baking, welding a bonding wire, dispensing a first layer of anti-reflection glue, baking, dispensing a second layer of anti-reflection glue, baking and testing. The anti-reflection glue is dispensed in a corresponding area of an RCLED lamp bead, so that a part capable of reflecting light in the RCLED lamp bead is shielded; the reflection effect of the RCLED lamp bead is effectively eliminated, the light spot emitting effect of the RCLED lamp bead is improved, and the light spots are clearer and more accurate. And the viscosity of the first layer of anti-reflection glue has certain flowability, so that the anti-reflection glue can be rapidly filled in a specified area, and the production efficiency is high. And the second layer of anti-reflection glue is relatively high in viscosity and flows slowly after glue dispensing, so that the glue dispensing precision is improved, and the anti-reflection glue can be effectively prevented from shielding a light-emitting hole of the RCLED chip. When the bonding wire is welded, the support is the first welding spot, and the PAD of the RCLED chip is the second welding spot, so that the radian of the bonding wire is lower, the lamp bead has a good appearance, the dispensing amount is reduced, and the production cost is saved.
Owner:PSG OPTO DEV CO LTD
Identification method for effective components in ginkgo leaf preparation
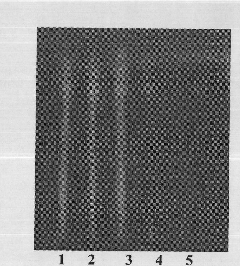
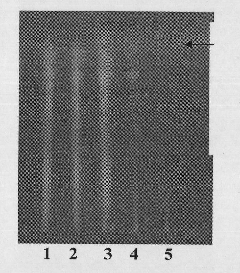
The invention discloses an identification method for the effective components in a ginkgo leaf preparation. The identification method comprises: (1) preparing a testing product: taking a proper amount of a ginkgo leaf preparation to be detected, adding n-butanol, extracting, filtering, evaporating the filtrate, and adding ethanol to the residue to dissolve so as to be adopted as the testing product solution; (2) preparing a reference substance solution: taking a proper amount of a ginkgo leaf reference preparation, and preparing the reference substance solution according to the step (1); and (3) carrying out thin layer chromatography: respectively sucking 1-10 [mu]lt of the testing product solution and the reference substance solution, respectively spotting on the same silica gel G plate, developing with an ethyl acetate, isopropyl alcohol, methanol and water mixture, taking out the thin plate, carrying out air drying, spraying a 1-5% aluminum chloride ethanol solution, placing in a 365 nm ultraviolet light lamp, viewing the fluorescence, and observing the spot. According to the present invention, with the identification method, the good separation effects of various effective components are good, the spots are clear, the Rf value is moderate, various effective components can be accurately identified, and the qualitative analysis and quality control are easily achieved.
Owner:HANGZHOU CONBA PHARMA
Pedicularis longiflora Rudolph.var.tubiformis formula granule, preparation method and detection method thereof
ActiveCN110075179AInconvenient to carryReduced stabilityComponent separationPharmaceutical non-active ingredientsDigestionBULK ACTIVE INGREDIENT
The invention relates to a pedicularis longiflora Rudolph.var.tubiformis formula granule, a preparation method and a detection method thereof. The formula granule is composed of pedicularis longifloraRudolph.var.tubiformis dry paste powder, silicon dioxide and sodium lauryl sulfate. The formula granule is prepared by performing digestion, spray drying and pelletizing on a single drug of the pedicularis longiflora Rudolph.var.tubiformis, and is convenient for a physician to carry out treatment based on syndrome differentiation and add and reduce according to syndromes; the detection method ofthe pedicularis longiflora Rudolph.var.tubiformis formula granule comprises performing thin layer chromatography identification by using a reference crude herb of the pedicularis longiflora Rudolph.var.tubiformis and a reference substance of luteolin, measuring the content of the main component luteolin by using high performance liquid chromatography, and detecting the content of tricin by using ultraviolet-visible spectrophotometry with the reference substance of the tricin as control; the detection method can reflect the quality of the pedicularis longiflora Rudolph.var.tubiformis formula granule comprehensively, guarantees complete reservation and stable content of the active ingredients of the pedicularis longiflora Rudolph.var.tubiformis formula granule, and makes the detection methodof the medicine quality more scientific and complete, and the medicine quality is better controlled.
Owner:青海普兰特药业有限公司
Quality detection method of pharmaceutical composition for relieving cough and asthma
ActiveCN112710797AShort detection cycleReduce testing costsPreparing sample for investigationTesting medicinal preparationsUrsolic acidPharmaceutical drug
The invention relates to a quality detection method of a pharmaceutical composition for relieving cough and asthma, wherein the pharmaceutical composition is composed of pinecones, cotton roots and loquat leaves; the detection method comprises identification of the active components pinecones, cotton roots and loquat leaves, and determination of oleanolic acid and ursolic acid content in loquat leaves. On the basis of the original quality standard, the identification method is optimized, the detection period is shortened, the detection cost is saved, and the identification for simultaneously detecting pinecones, cotton roots and loquat leaves by using the same thin-layer plate is obtained; in addition, by adding the detection of heavy metals and harmful elements and adding thedetermination of oleanolic acid and ursolic acid content in loquat leaves, the effective components of the medicine are controlled from qualitative detection to qualitative and quantitative detection. The detection method is high in accuracy and good in stability and can be effectively used for controlling the quality of the cough and asthma relieving medicine combination and improving and perfecting the quality standard.
Owner:贵州大隆药业有限责任公司
Thin-layer chromatographic detection method of curcuma zedoary in Fushengkang tablet
InactiveCN107328890ASolve gelatinizationSolve stickinessComponent separationThin layer chromatographicColor tests
The invention discloses a thin-layer chromatographic detection method of curcuma in Fushengkang tablets. This method uses ethyl acetate to extract the volatile oil in Fushengkang as the test solution; and uses the same method to process the reference medicinal material Curcuma to make a reference medicinal solution; petroleum ether (60-90°C)-ethyl acetate-glacial acetic acid is used as Developing agent, use silica gel thin-layer plate to develop, spray with newly prepared vanillin test solution, heat at 105°C until the spots are clear in color; If there are no spots, it means that the finished product of Fushengkang contains zedoary ingredients; and if the chromatogram of the test product does not show the main spot of the same color at the position corresponding to the chromatogram of the reference medicinal material, it means that the finished product of Fushengkang does not contain zedoary ingredients. The present invention uses ethyl acetate to reflux heat the sample instead of using ether to sonicate the sample, which solves the problems of sample gelatinization, adhesion, and difficult filtration, and reduces the potential harm to human health; the color development result is accurate: the spots are clear and reproducible Good performance and high accuracy.
Owner:青岛华仁太医药业有限公司
Gentiana urnula formula granule, preparation method and detection method thereof
InactiveCN110075155AHigh content of impuritiesInconvenient to carryComponent separationPharmaceutical non-active ingredientsPolyethylene glycolThin layer
The invention relates to a gentiana urnula formula granule, a preparation method and a detection method. The formula granule is composed of dry gentiana urnula paste powder, dextrin and polyethylene glycol. The gentiana urnula formula granule is prepared from a single medicine of gentiana urnula through extracting, concentrating, drying and pelletizing; treatment based on syndrome differentiationby a physician can be facilitated, the dosage is increased or decreased based on the syndrome; the detecting content is to identify gentiana urnula glycoside a through the thin layer chromatography, and detect the content of the gentiana urnula glycoside a in the formula granule through the liquid chromatography. By adopting the method, under different thin layer plates, temperature and humidity conditions, the spots on chromatograms are clear, the separation is in accord with the requirements, the Rf value is moderate, and better identification chromatography can be obtained under different conditions; a verification test shows that the method provided by the invention is good in reproducibility and durability, the liquid chromatography is adopted to detect the content of the gentiana urnula glycoside a, the result is accurate and reliable; the method is simple, good in repeatability, and can be used for effectively controlling the quality of the gentiana urnula formula granule.
Owner:青海普兰特药业有限公司
Detection method for discriminating baicalin and chlorogenic acid thin layer in compound honeysuckle flower particles
InactiveCN109709256ASimple preparation stepsStrong specificityComponent separationChlorogenic acidPolyamide
The invention provides a detection method for discriminating a baicalin and chlorogenic acid thin layer in compound honeysuckle flower particles. The method comprises the following steps that 1) a tested object solution, a negative control solution, a baicalin contrast solution and a chlorogenic acid contrast solution are prepared, a developing solvent and a developer are prepared, and the developing solvent comprises ethyl acetate, methanol and acetic acid in the volume ratio of (1-7): (2-5): (7-15); 2) thin layer chromatography is carried out by taking a polyamide thin film as a thin layer plate, dropping the tested object solution, negative control solution, baicalin contrast solution and chlorogenic acid contrast solution on the thin layer plate, placing the thin layer plate dropped with samples in a developing cylinder saturated with the developing agent to implement development, removing the thin layer plate, and air drying; and 3) chlorogenic acid and baicalin are inspected in UV and daylight respectively. The method can be discriminate thin layers of the baicalin and chlorogenic acid in the compound honeysuckle flower particles specifically.
Owner:XIAN DAQING PHARMA FACTORY JINHUA ENTERPRISE GROUP CORP
Method for identifying medlar and common cnidium fruit in Qirong tablets
ActiveCN101797302ASimple and fast operationEasy to separateComponent separationPill deliveryCnidiumBiology
The invention relates to a method for identifying medlar and common cnidium fruit in Qirong tablets. The qualitative identification of the medlar and common cnidium fruit in the Qirong tablets is performed by adopting thin layer chromatography. Tests prove that the method for identifying the medlar and the common cnidium fruit in the Qirong tablets has the advantages of simple and convenient operation, excellent separation effect, high sensitivity and clear spots. Through negative control, the interference is absent. The method is proved to have specificity and durability, so the method can be listed as quality standard of the Qirong tablets.
Owner:宁夏启元国药有限公司
Quality control method for Jinfozhitong pill
ActiveCN1588081ALow backgroundClear spotsComponent separationColor/spectral properties measurementsPaeoniflorinCITRUS MEDICA FRUIT
The invention discloses a quality control method for gold Buddha analgetic pill, it increases thin layer chromatography incrimination method of white peony root, corydalis tuber based on original fingered citron incrimination. It also uses high efficiency liquid chromatography incrimination method to detect the content of the paeoniflorin. The control method of the invention upgrades the accuracy, effectiveness of control, and it realizes the effective control of the product.
Owner:GUANGZHOU BAIYUNSHAN ZHONGYI PHARMA COMPANY
Detection method of babaowudan cinnabar stick
The invention provides a detection method of babaowudan cinnabar stick. The detection method comprises a borneol detection method and a bufotoxin detection method. Diethyl ether is adopted for extraction and taken as a test solution for detection, then dregs of decoction are taken as samples for a bufotoxin identification experiment, part of impurities in bufotoxin identification are removed, and further, the use amount of the samples is reduced. When amethanol extracting solution is taken as a test solution, more serious thin-layer chromatography interference is produced after development, and bufotoxin detection is affected; the methanol extracting solution is concentrated and passes a neutral aluminum oxide column, methanol is used for elution, a large quantity of impurities can be removed, and thin-layer chromatography interference can be reduced. A developer for bufotoxin detection adopts a toluene-acetone developer, the Rf (retardation factor) value is moderate, and spots are clear.
Owner:ANHUI MOYAO PHARMA
A quality detection method for a combination of medicines for treating cough, dyspnea and chest fullness
ActiveCN109374815BShort heating timeShorten drying timeComponent separationNeurological disorderPharmacology
The invention relates to a quality detection method of a medicinal composition for treating cough, dyspnea and chest fullness. The composition consists of pine cones, cotton roots and loquat leaves. The quality detection method comprises: active ingredients pine cones, cotton roots, loquat Identification of leaves, determination of free gossypol residues in cotton roots. Free gossypol is a kind of polyphenolic hydroxyl bis-naphthaldehyde compound, which is a toxic substance that can cause redness, swelling and bleeding, nervous disorders, loss of appetite, weight loss, and affect fertility. On the basis of the original quality standard, the present invention optimizes the identification method, increases the identification of loquat leaves, and measures the residual amount of free gossypol in cotton roots, so that the quality monitoring level of the drug combination for treating cough, dyspnea and chest fullness has been significantly improved. Great improvement, the quality detection method has high accuracy and good stability, and can be effectively used for the quality detection of drug combinations for treating cough, asthma and chest fullness, and improves the quality standard.
Owner:贵州大隆药业有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com