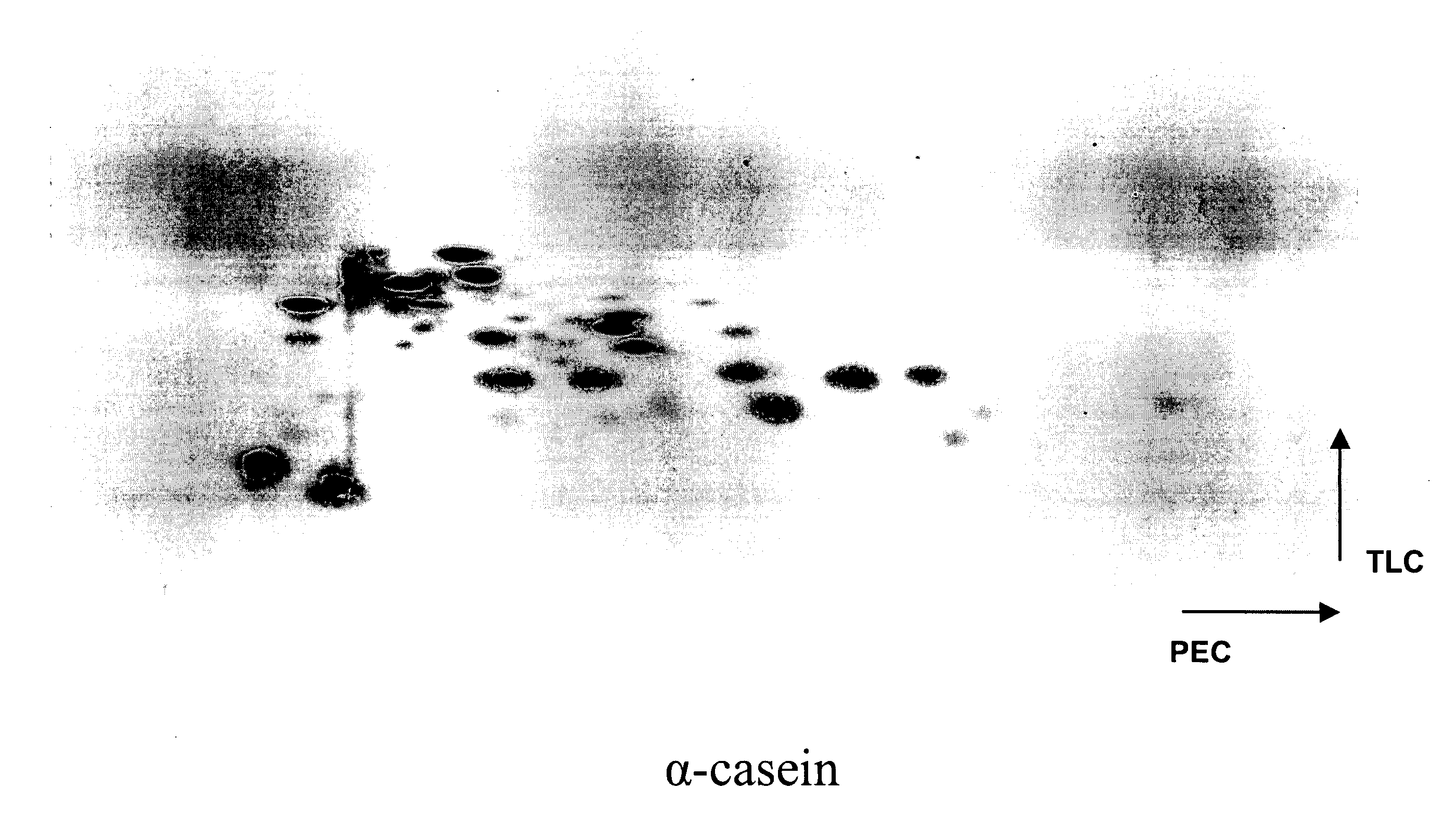
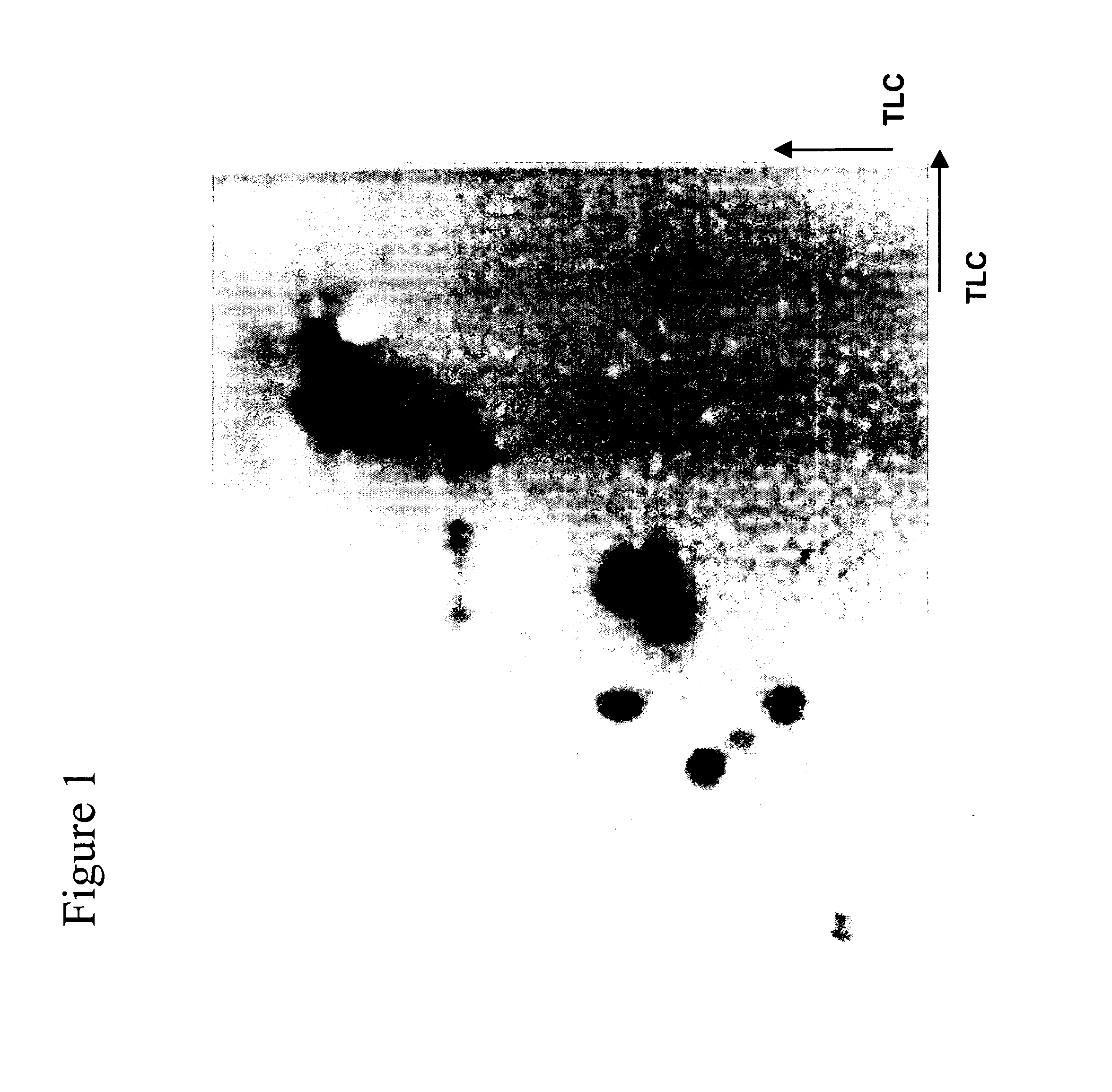
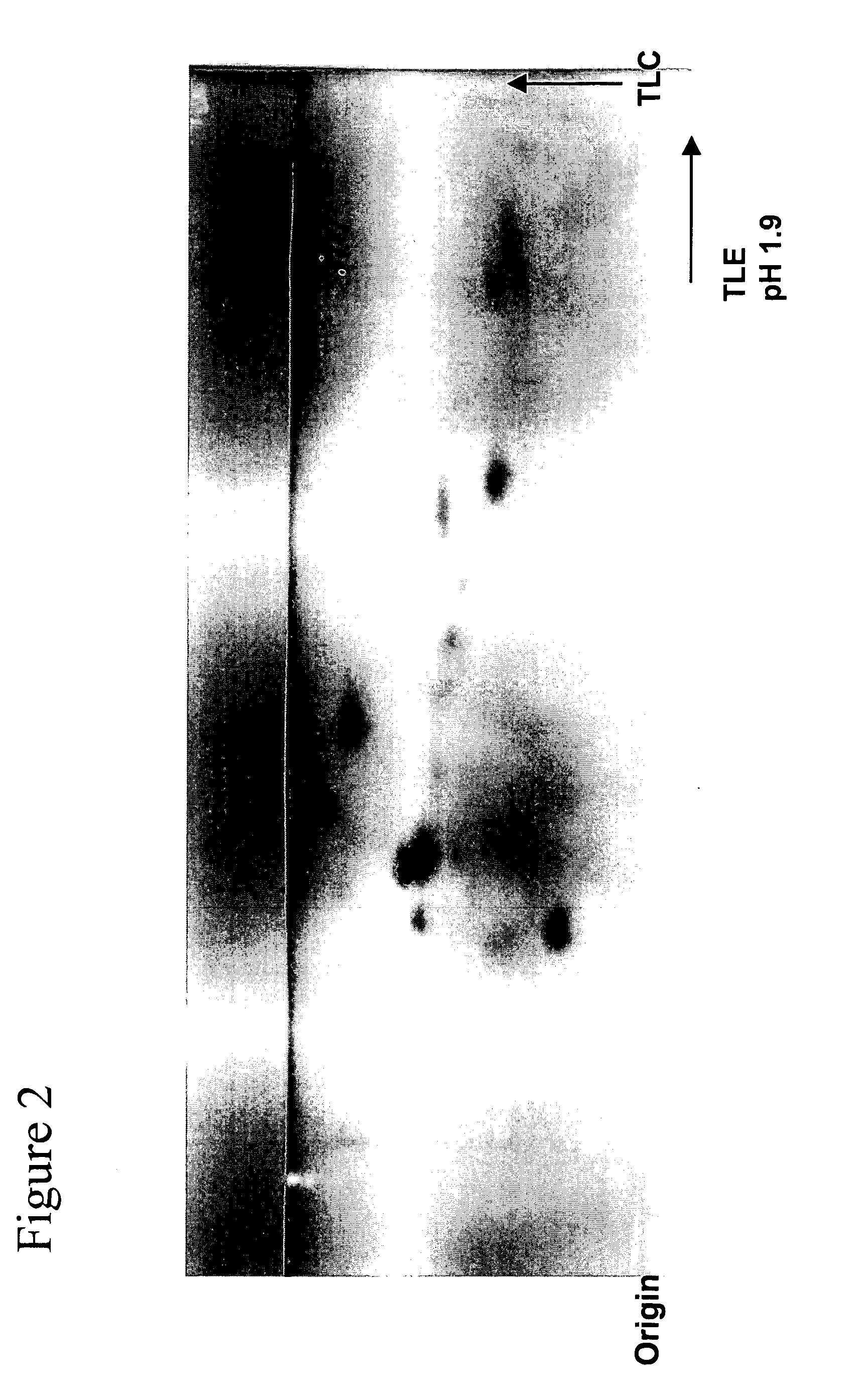
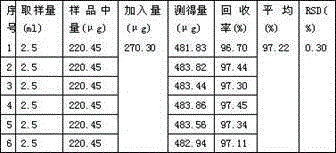
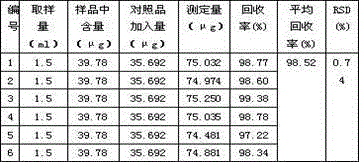
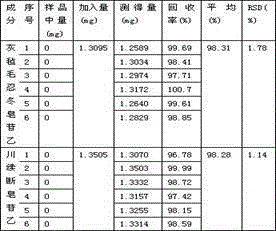
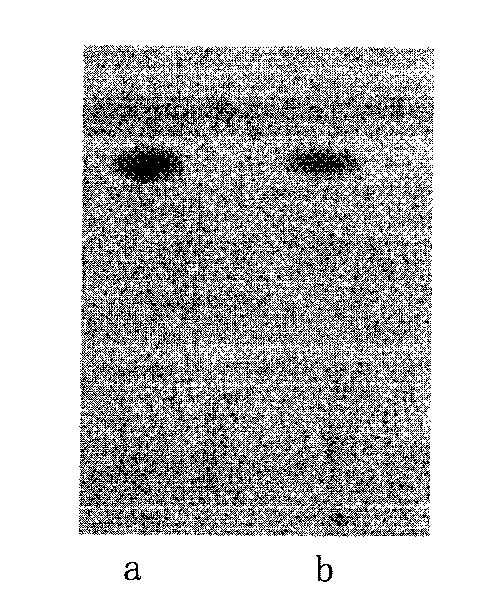
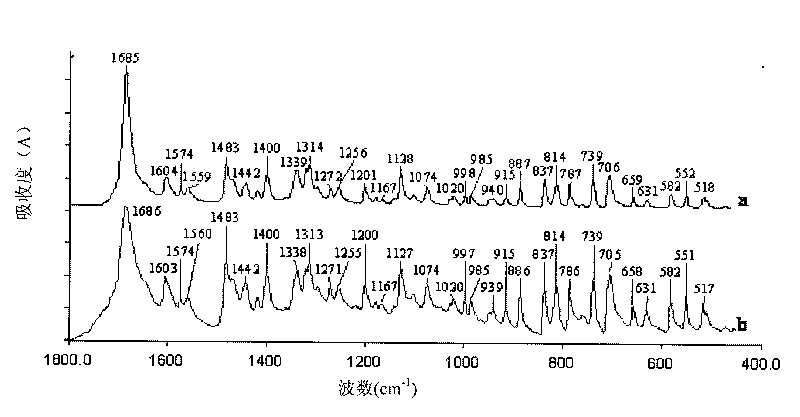
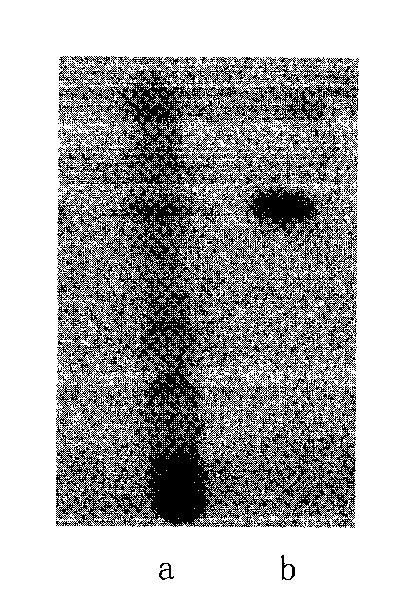
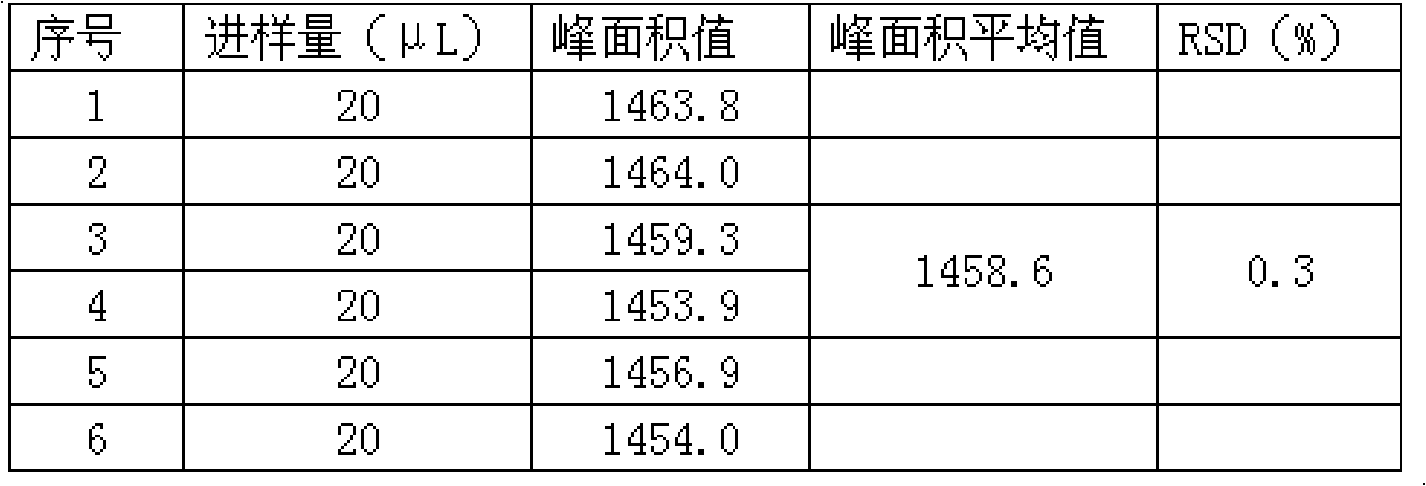
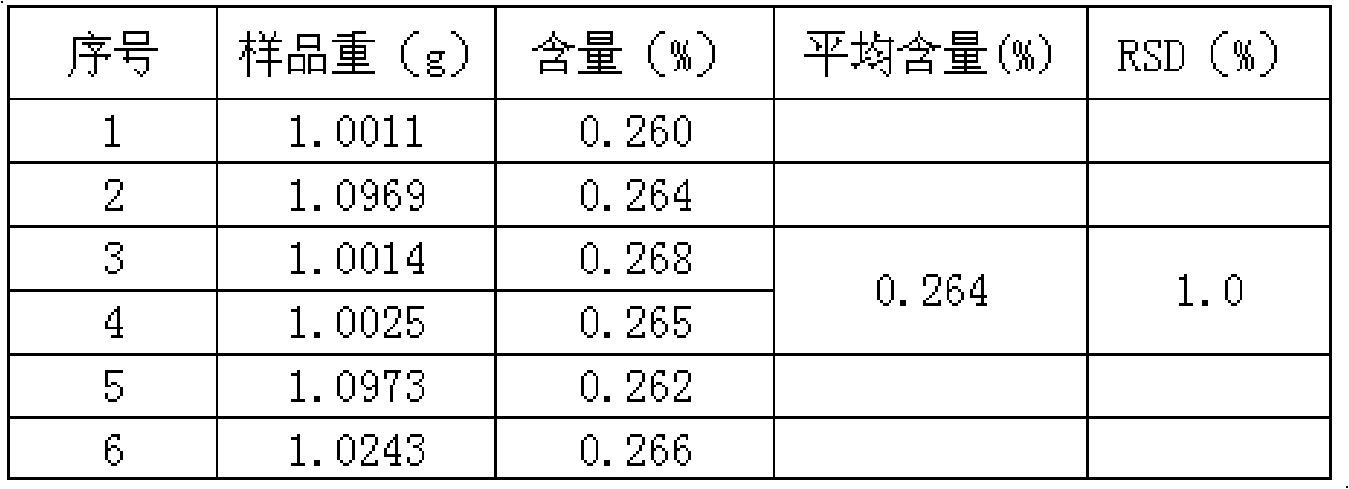
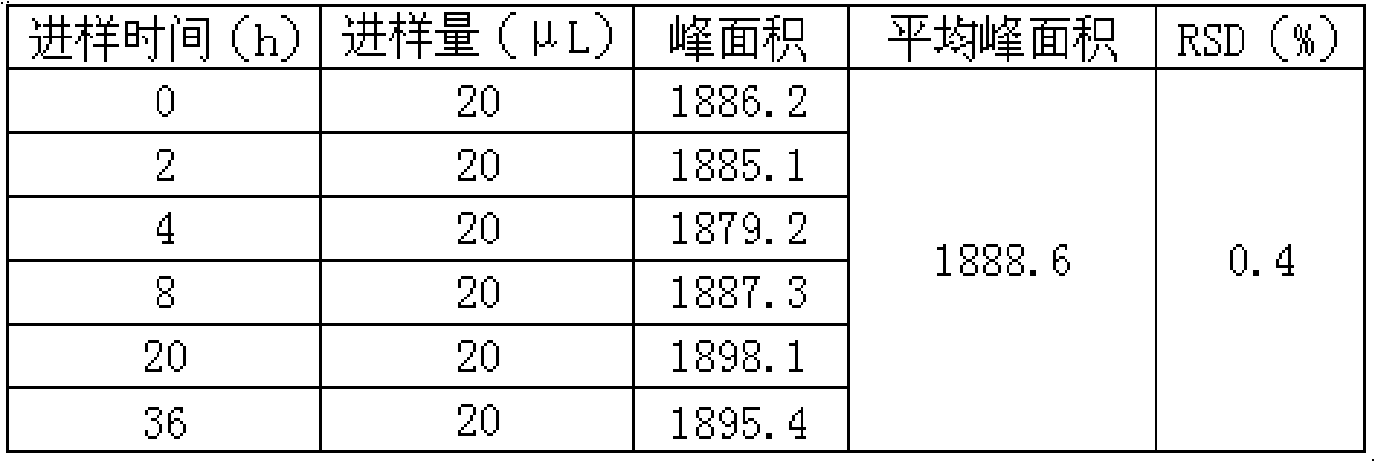
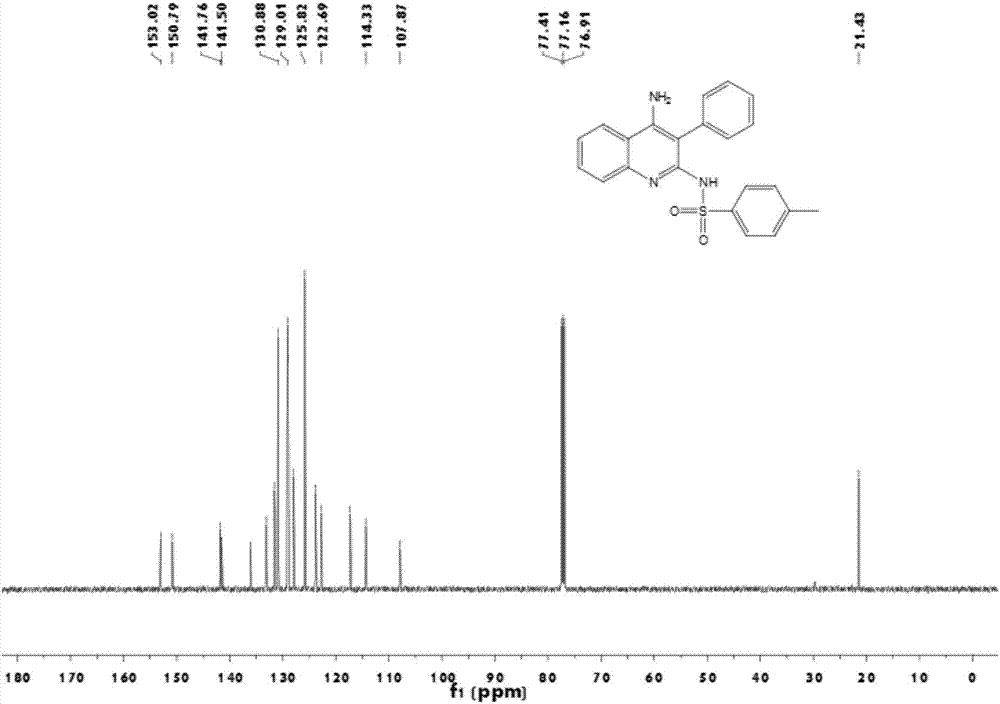
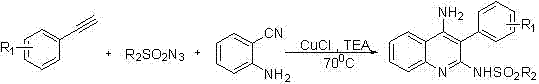
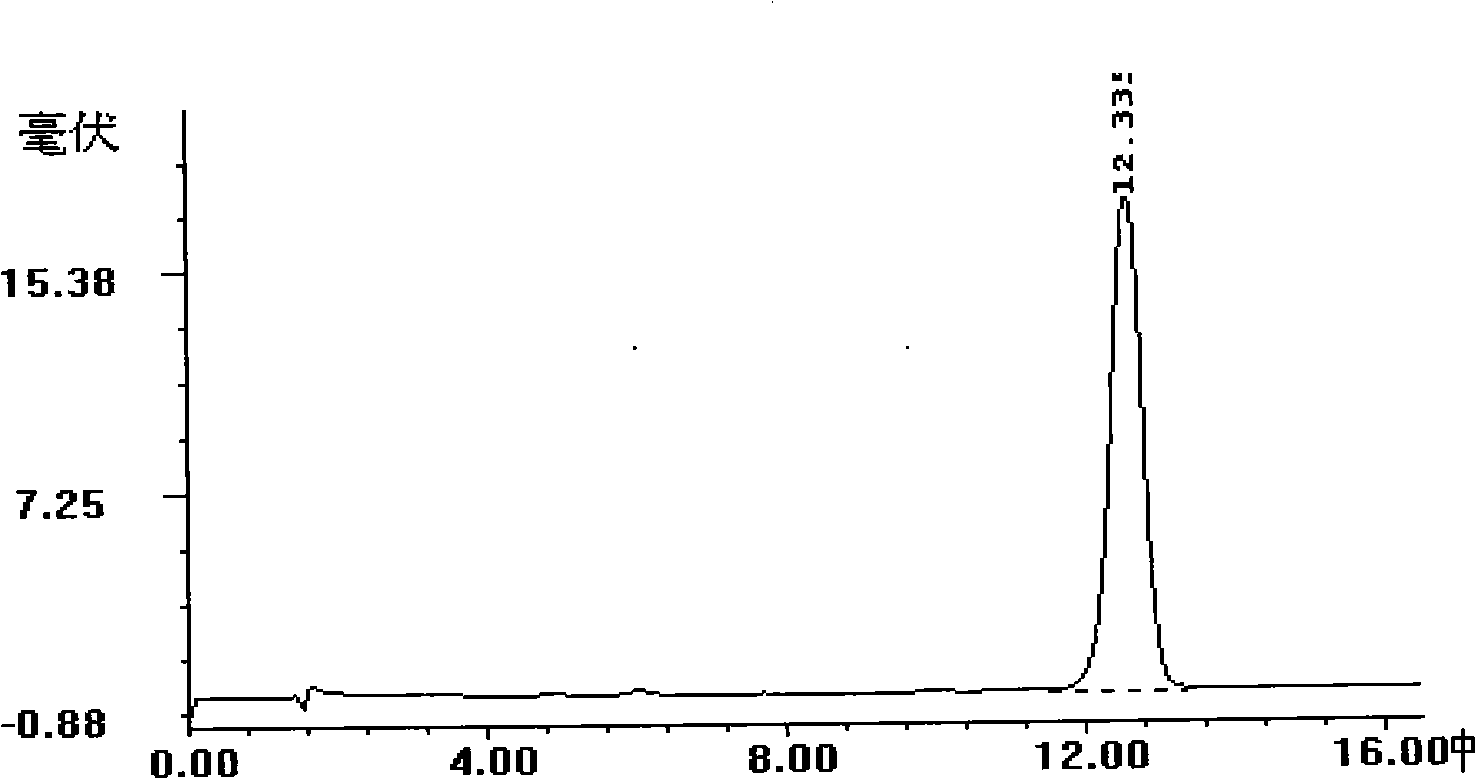
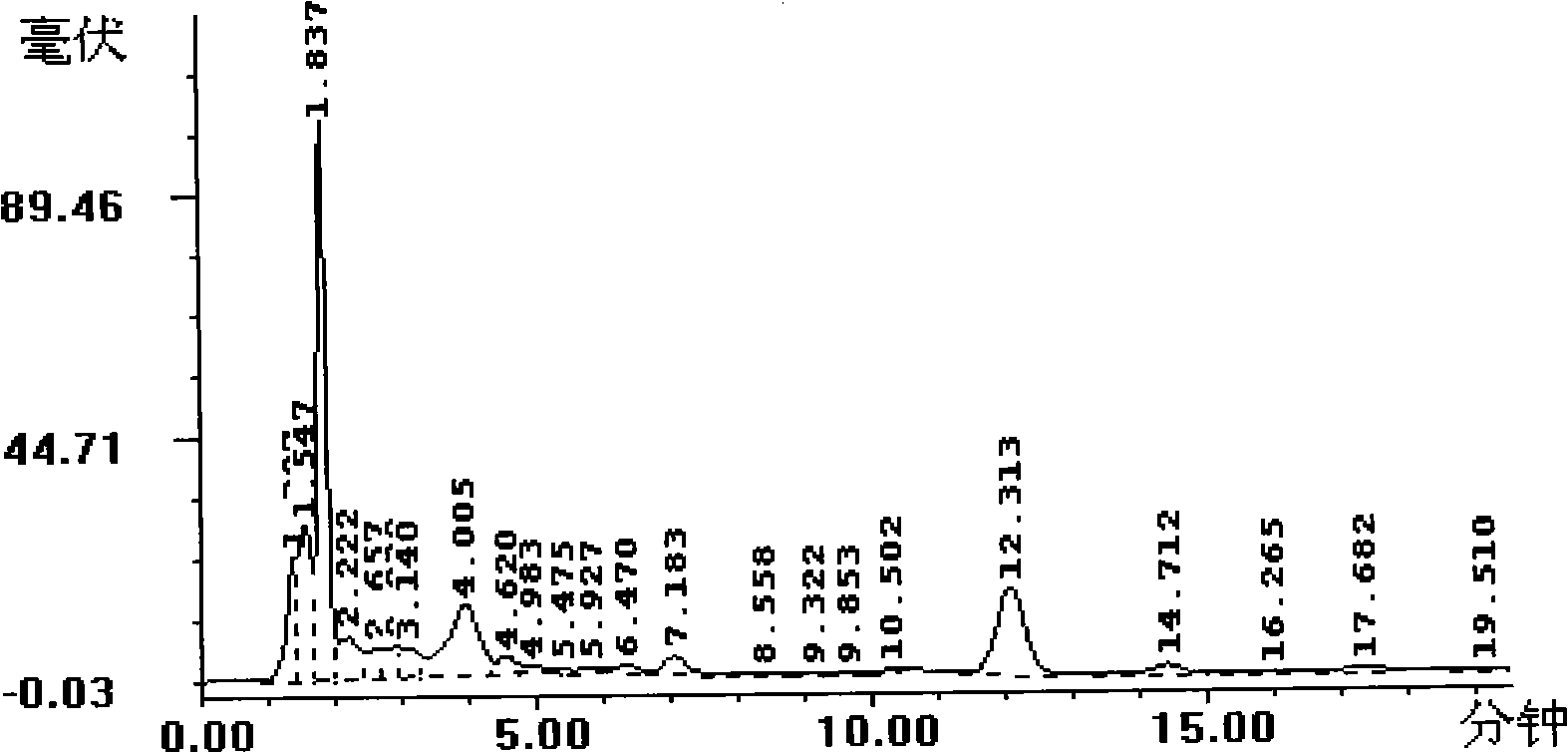
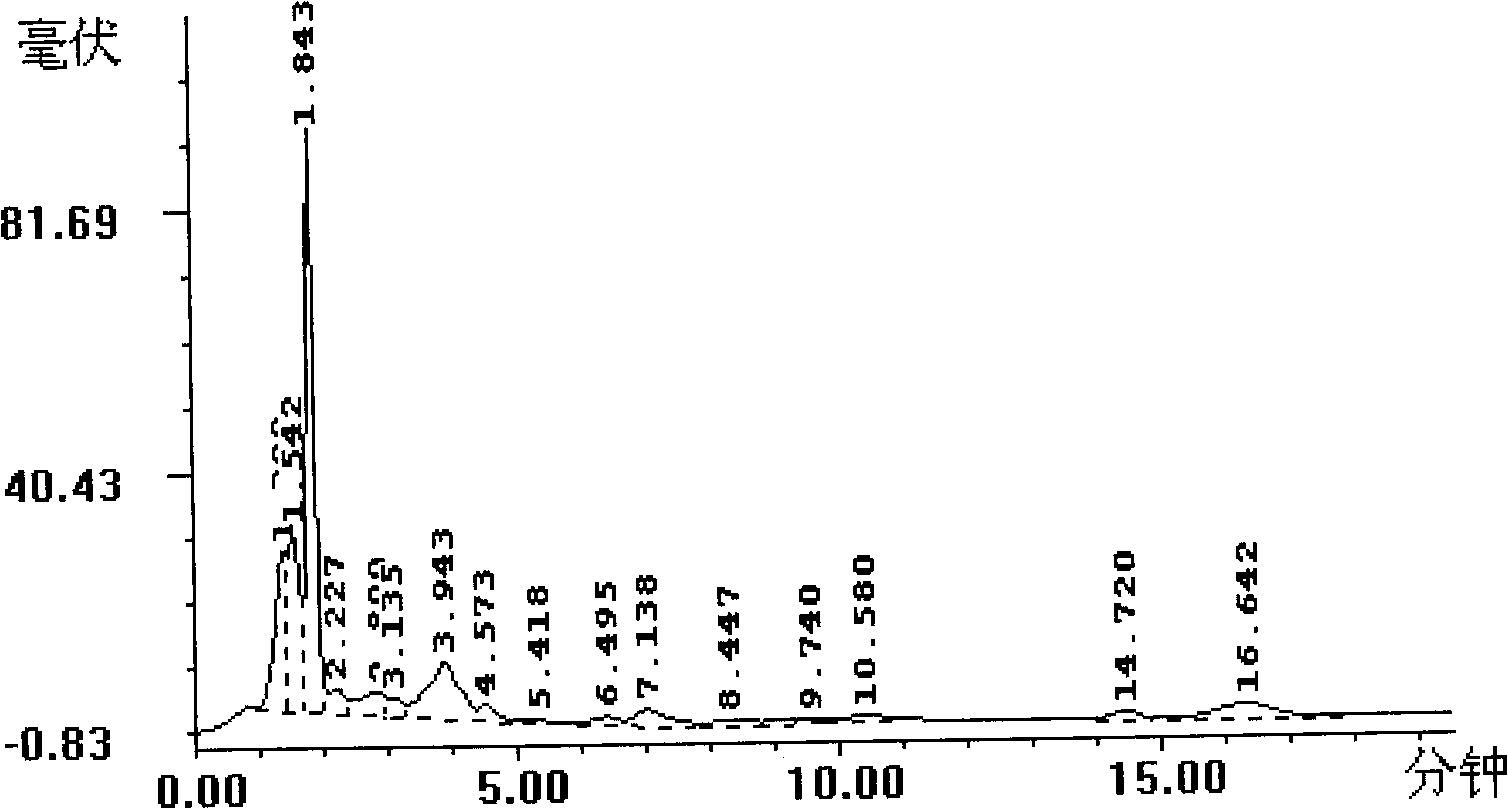
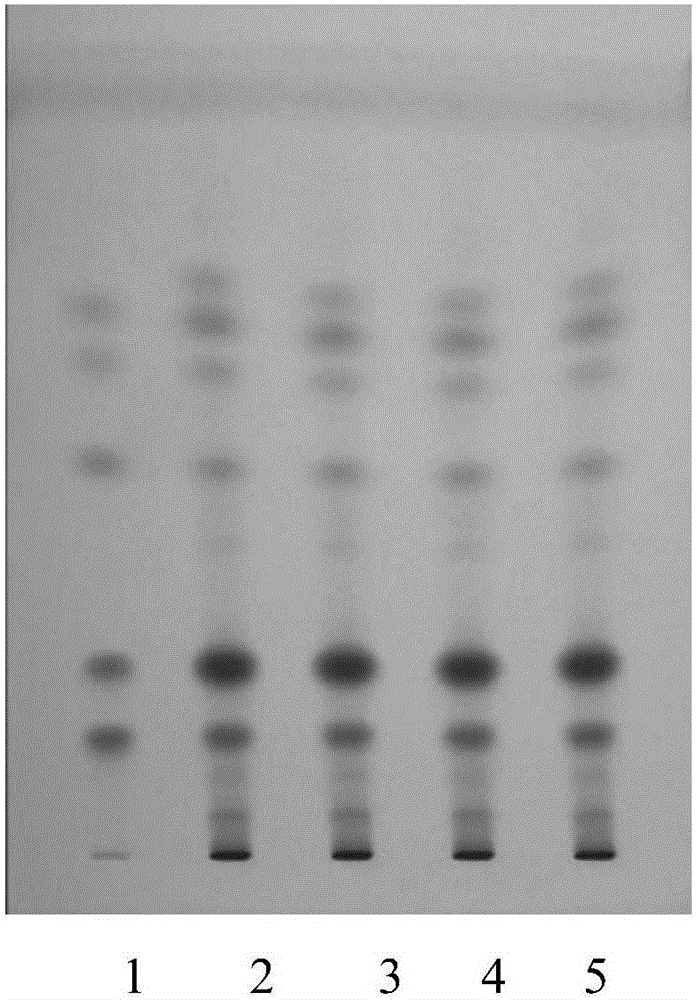
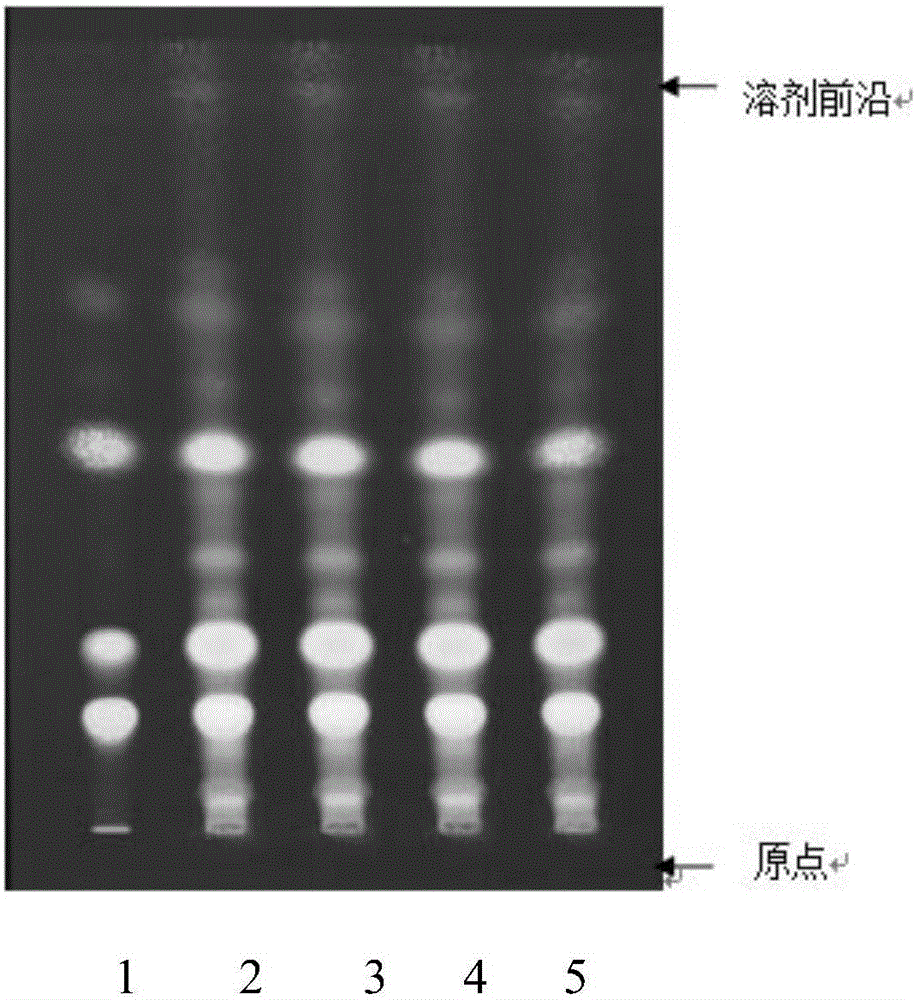
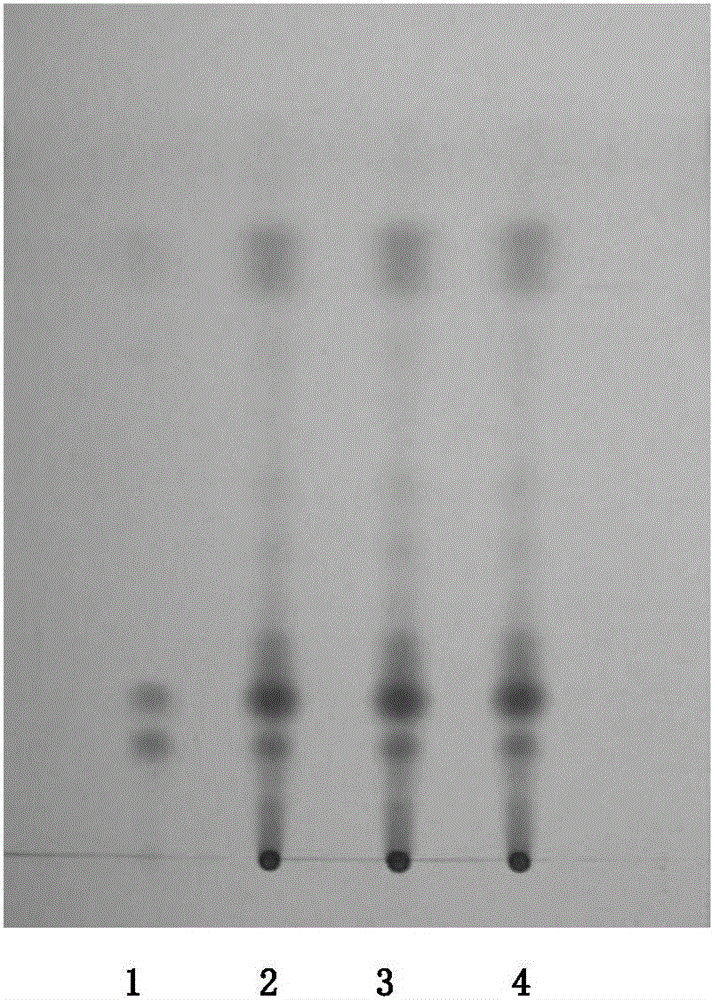
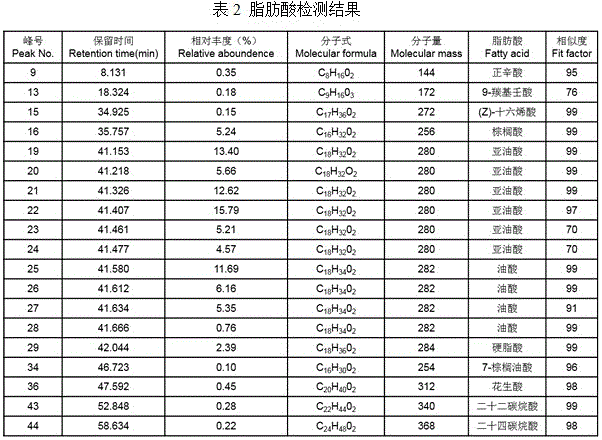
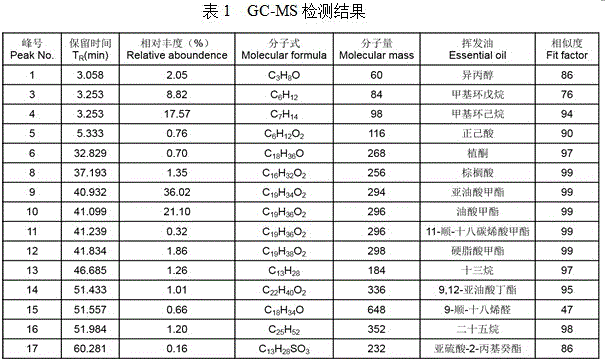
Patents
Literature
224 results about "Thin layer chromatogram" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Planar electrochromatography/thin layer chromatography separations systems
InactiveUS20070187243A1Facilitate fractionationReduces sample consumptionSludge treatmentComponent separationChromatographic separationStationary phase
A method for separating a sample comprising a plurality of compounds includes loading a sample onto a planar stationary phase, said sample comprising a plurality of compounds to be separated; and, while retaining the sample on the planar stationary phase, subjecting the sample to planar electrochromatographic separation in a selected direction using a first mobile phase; and subjecting the sample to a chromatographically-based separation in a direction other than that of the direction used for planar electrochromatographic separation using a second mobile phase. Separation is optionally followed by direct detection of analytes using mass spectrometry (MS). These three dimensions of separation are orthogonal to one another, providing for improved resolution of the analytes.
Owner:INCHROMATICS
Preparation and quality detection method of compound prescription cortex phellodendri chinensis fluid
InactiveCN104398642AAccurate massAccurate quality inspectionAntibacterial agentsComponent separationBerberis empetrifoliaForsythia
The invention discloses preparation and quality detection method of compound prescription cortex phellodendri chinensis fluid. The quality detection method comprises the identification of forsythia fruit, cortex phellodendri, honeysuckle and dandehon herb, the checking of lonicerae flos, and the content determination of forsythin and berberine hydrochloride. According to the determination method, spots with the same colors are formed in a qualified test article thin-layer chromatogram and in corresponding positions of a reference substance thin-layer chromatogram, and negative control is interference-free, and the method is good in selectivity, simple and accurate. The checking method of lonicerae flos adopts a high performance liquid chromatography, is good in specificity, and can distinguish the situation that lonicerae flos is adopted in the compound prescription cortex phellodendri chinensis fluid to pretend honeysuckle flower to charge material. The content determination of forsythin and berberine hydrochloride is good in linear relation and high in recovery test accurancy, has good repeatability and stability, can accurately and strictly detect the quality of the compound prescription cortex phellodendri chinensis fluid, and guarantees a high quality standard level of the compound prescription cortex phellodendri chinensis fluid.
Owner:SHANDONG HANFANG PHARMA
Method for detection of illegally added chemical in traditional Chinese medicine preparation
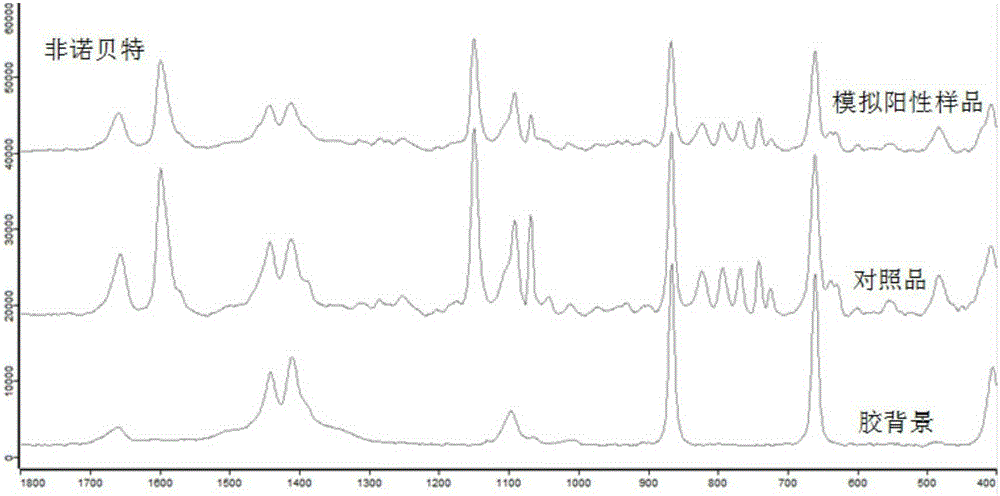
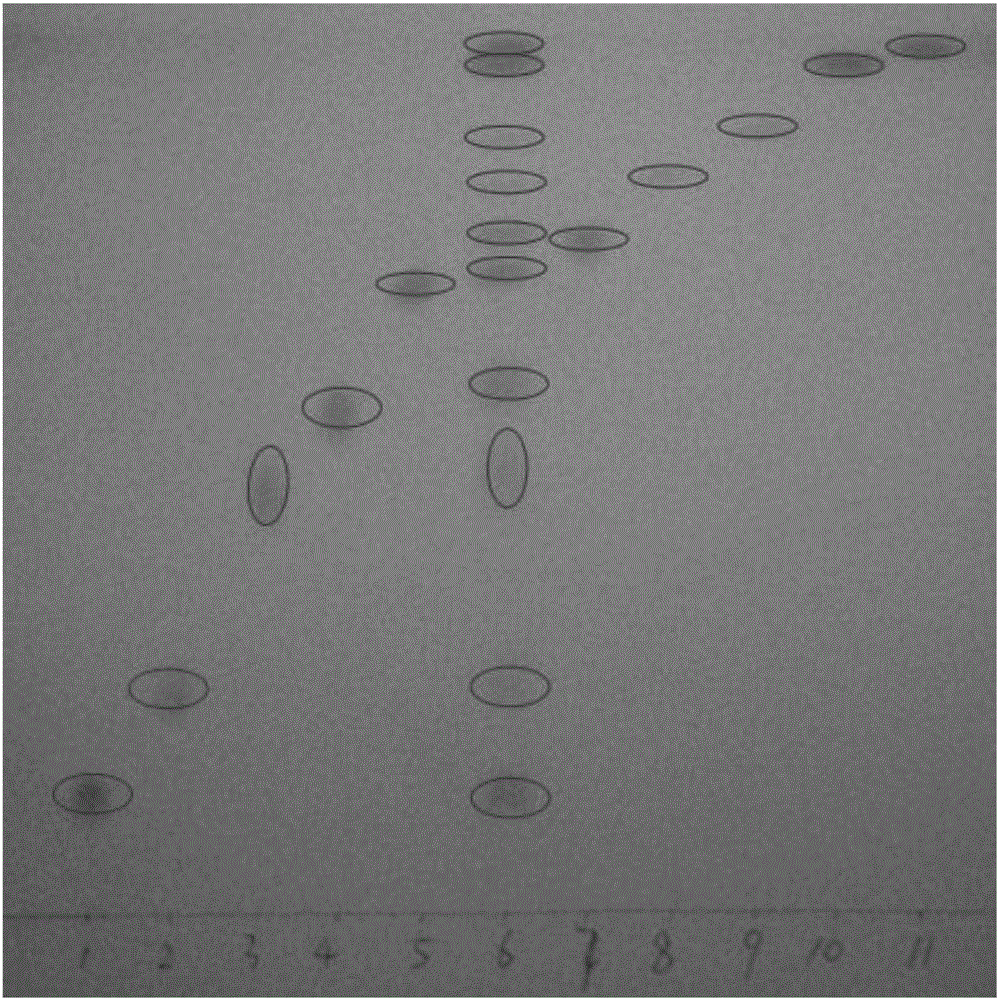
The invention discloses a method for detection of an illegally added chemical in a traditional Chinese medicine preparation, which comprises the following steps: (1) carrying out thin-layer chromatography simultaneously for both sample solution containing a traditional Chinese medicine preparation to be detected and a reference substance of a chemical suspected of being illegally added; and (2) if the thin-layer chromatogram of the sample solution containing the traditional Chinese medicine preparation to be detected has spots in positions corresponding to the positions of the spots of the reference substance, performing infrared spectrum analysis for both silica gel in the positions with the spots of the traditional Chinese medicine preparation to be detected and silica gel in the positions with the spots of the reference substance, and then determining whether the traditional Chinese medicine preparation to be detected contains the chemical suspected of being illegally added. The invention can rapidly and accurately judge whether the traditional Chinese medicine preparation contains the illegally added chemical, and provides a novel method and means for detection of present adulteration of the traditional Chinese medicine preparation.
Owner:中国人民武装警察部队药品仪器检验所
Determination method for saturated hydrocarbon, arene, colloid, asphalt content in oil product
InactiveCN1763522AHarm reductionImprove consistencyComponent separationData treatmentThin layer chromatogram
The invention discloses a detecting method of saturated hydrocarbon, aromatic hydrocarbon, colloid and asphaltene content in one oil, which comprises the following steps: diluting the 1-2 weight sample through 45-90 weight benzeneú” preheating for half a hour through thin-layer chromatograph to do blank scan; setting the drier temperature between 100 and 120 deg.c; extracting 1.0ª–l sample through microscale injector to drip on the chromarod for 5 times; moving the chromarod at the first, second and third developing tank; connecting the host and disposer to scan the chromarod on the hydrogen flame; displaying chromatogram, chromatogram peak area and hydrocarbon, aromatic hydrocarbon, colloid, asphaltene content on the data disposer automatically, which realizes the detection of single or mixing oil.
Owner:PETROCHINA CO LTD
Method for testing quality of perfoliate knotweed medicinal materials
InactiveCN101549021AImprove quality inspection standardsHigh precisionSenses disorderAntipyreticMedicinal herbsTest quality
The invention discloses a method for testing quality of perfoliate knotweed medicinal material, which includes part or whole of description, identification, examination and content measurement. The aforementioned identification includes thin-layered chromatography of taking quercetin reference substance and / or quercetin-3-O-beta-D-n butyl glucuronic acid reference substance as contrast, the content measurement measures the content of quercetin in medicinal materials, which is indicated as a high performance liquid chromatography of taking quercetin as contrast and the organic facies : aqueous phase as 10-40 : 90-60. Compared to the existing technology, the invention perfects the quality detecting standard of perfoliate knotweed medicinal materials, recuperates the deficiency of the existing quality detecting technology, which makes this quality detecting technology more scientific and reasonable, and ensures its security and validity in clinical application.
Owner:GUIZHOU NORMAL UNIVERSITY
Method for detecting contents of glyceride and free fatty acid in biodiesel
ActiveCN104237447AEfficient detectionDetermination of contentComponent separationBiodieselMonoglyceride
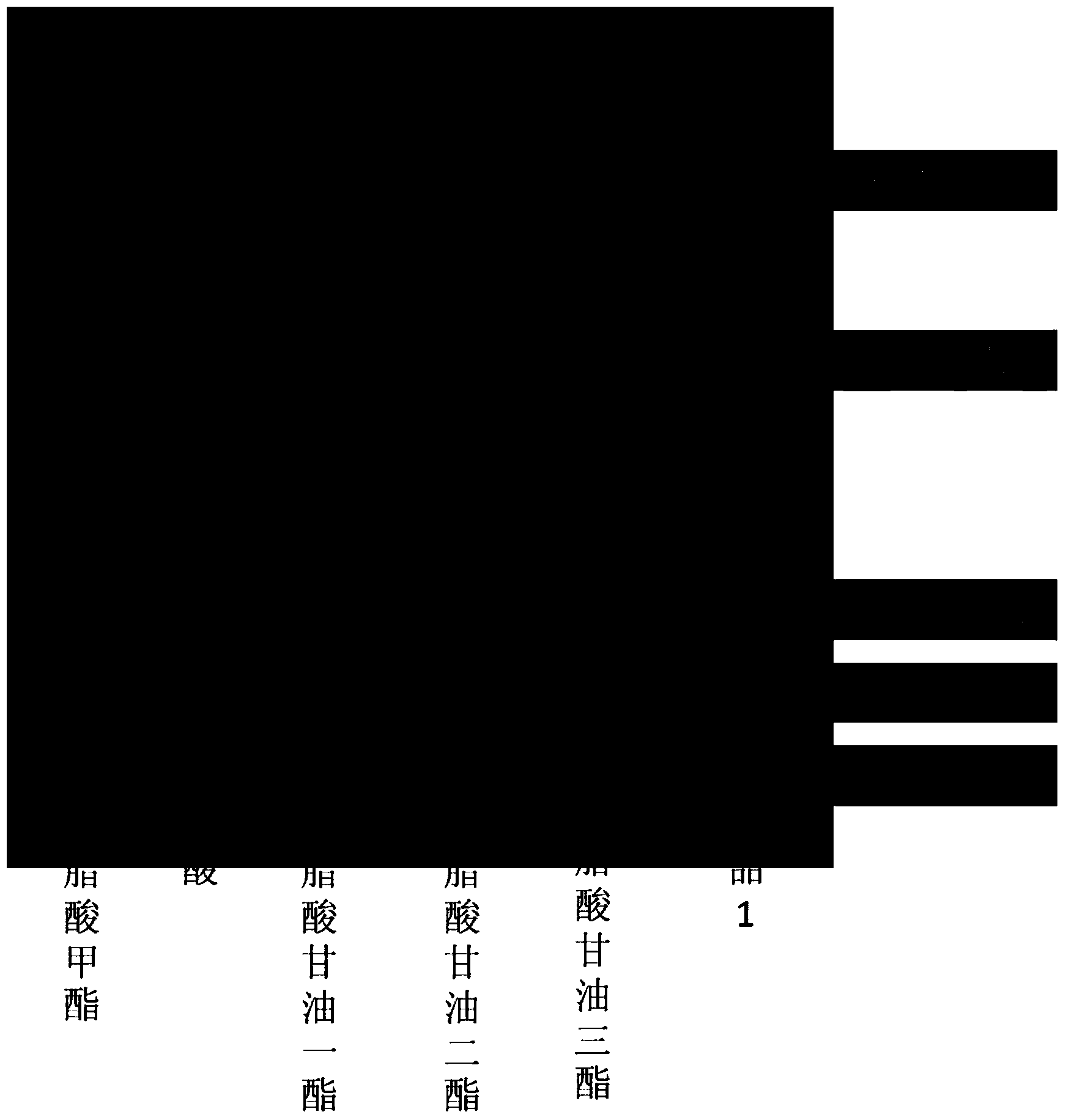
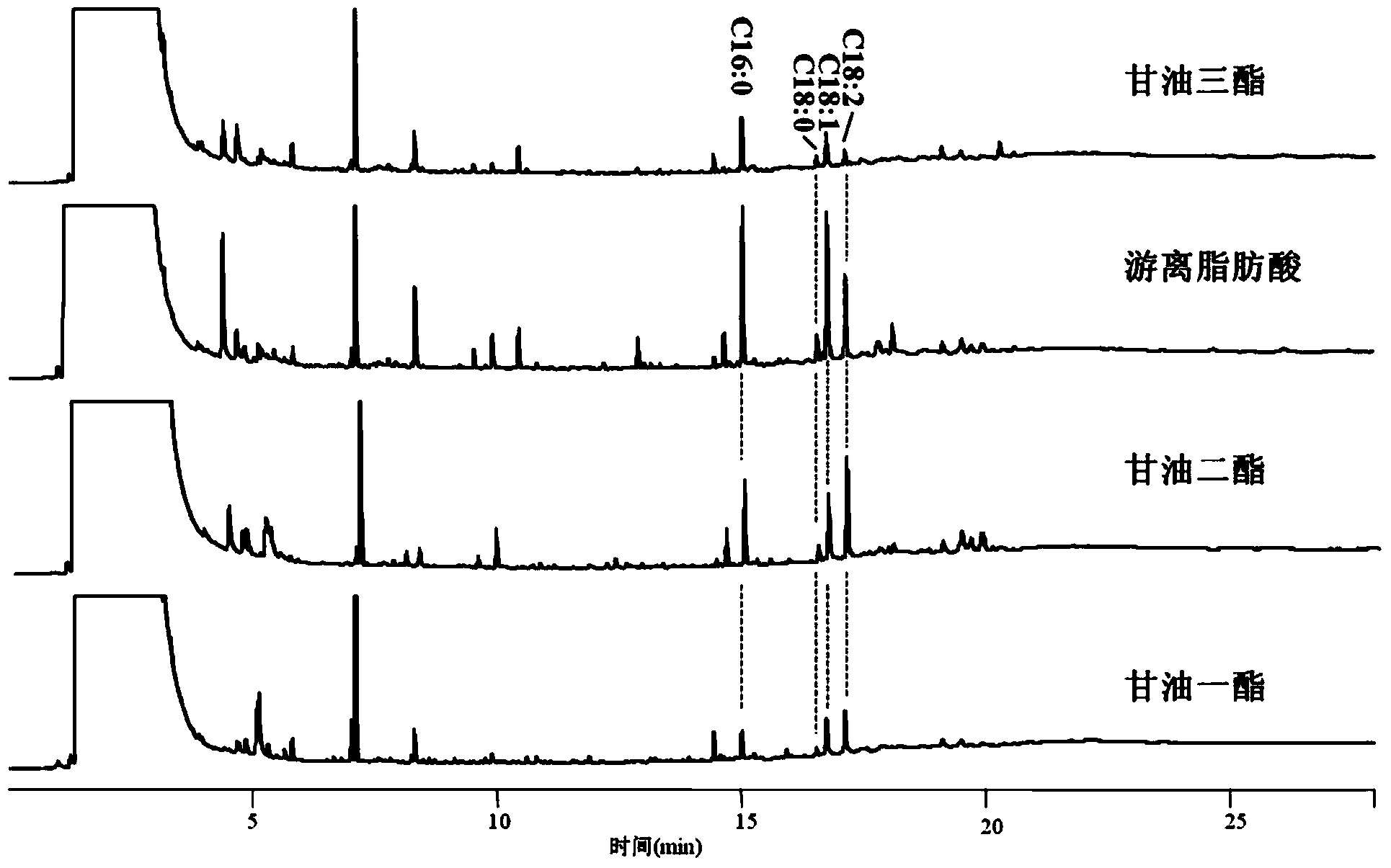
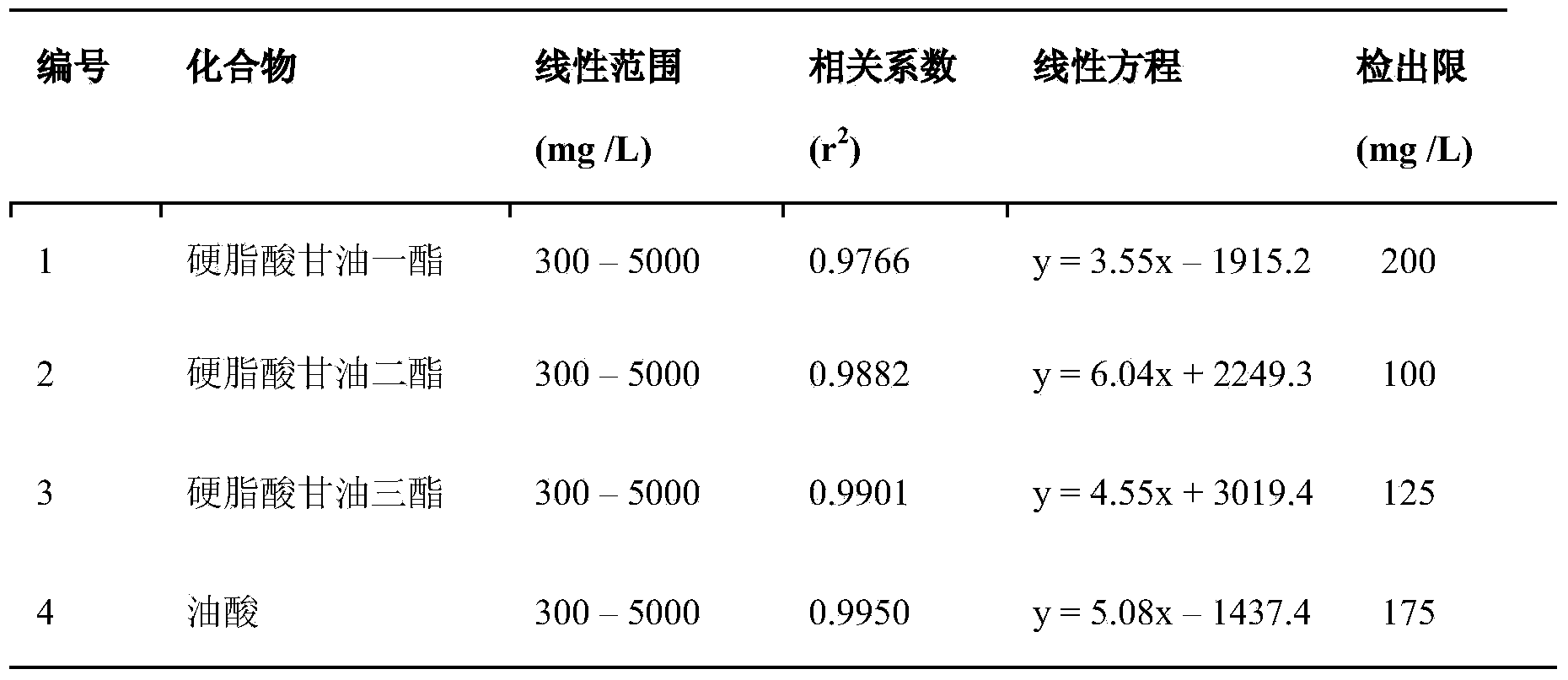
The invention discloses a method for detecting the contents of glyceride and free fatty acid in biodiesel. The method comprises the following steps: diluting a biodiesel sample, carrying out thin layer chromatograph with an aluminum matrix silica gel plate; with n-hexane, acetic ether and formic acid mixed solvent as a developer, developing with iodine steam, wherein the volume ratio of the n-hexane, acetic ether and formic acid is 90:10:2; after separating, cutting off the spots of monoglyceride, diglyceride, glycerin trilaurate and free fatty acid; putting the cut spots and carbinol solution of three methyl hydrogen trioxide into a sample cup; putting the sample in a cracker; placing the cracker at a GC sample feeding opening, and feeding the sample when the temperature of the cracker reaches 300-450 DEG C; carrying out gas chromatograph detection to obtain the gas chromatogram of the to-be-detected sample; comparing the gas chromatogram with the standard curves of monoglyceride, diglyceride, glycerin trilaurate and free fatty acid and calculating to obtain the contents of glyceride and free fatty acid in the to-be-detected sample. The method is simple in operation, accurate in quantization and comprehensive in information and has profound significance on quality control of the biodiesel.
Owner:仕宝(天津)技术检测有限公司
Method for rapidly and simultaneously detecting ten adulterated components in lipid lowering Chinese patent medicine
ActiveCN105241861AStrong specificityEasy to operateComponent separationRaman scatteringLipid formationSurface-enhanced Raman spectroscopy
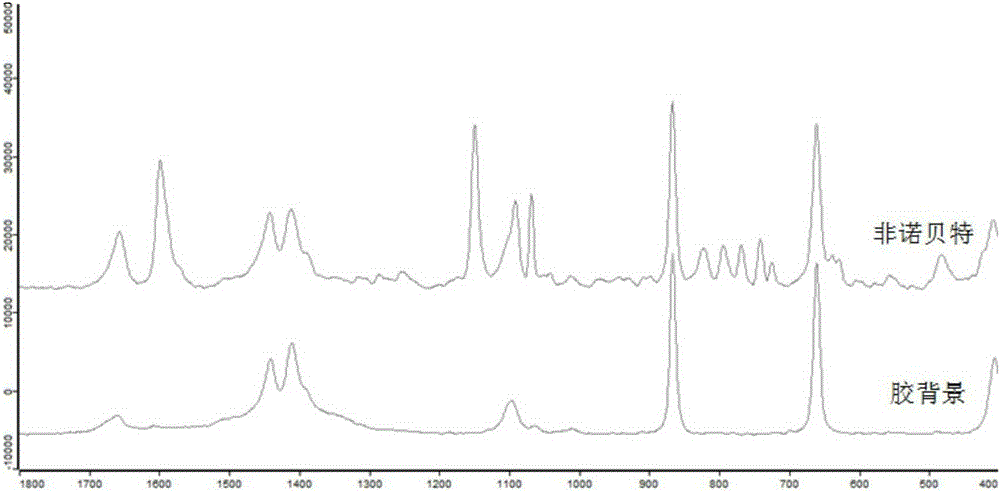
The invention provides a method for rapidly and simultaneously detecting ten adulterated components in a lipid lowering Chinese patent medicine. Chromatographic conditions for separation ten adulterated components in the lipid lowering Chinese patent medicine and surface-enhanced Raman spectroscopic detection conditions are rapidly and simultaneously found through a large amount of repeated experiments by adopting a thin layer chromatograph and surface enhanced Raman spectroscopy combination technology, so thin layer chromatograph and surface enhanced Raman spectroscopy combination conditions provided by the invention are suitable for detecting the low concentration adulteration of the lipid lowering Chinese patent medicine and are also suitable for detecting single adulteration or multiple simultaneous adulteration of the ten components, so troubles of every component condition finding needed by simultaneous detection of multiple adulterated components in the prior art are avoided. The method is simple to operate, is suitable for onsite direct detection, and separation and detection conditions provided by the invention have the advantages of simplicity, fastness, high sensitivity and strong specificity.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for preparing and purifying water-soluble cyanine dye (Cy5.5)
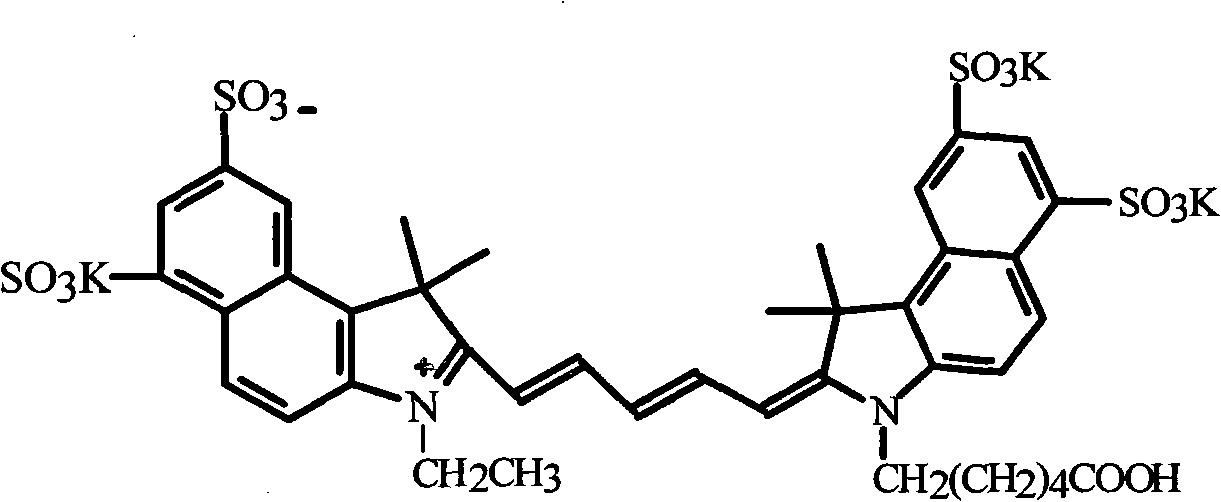
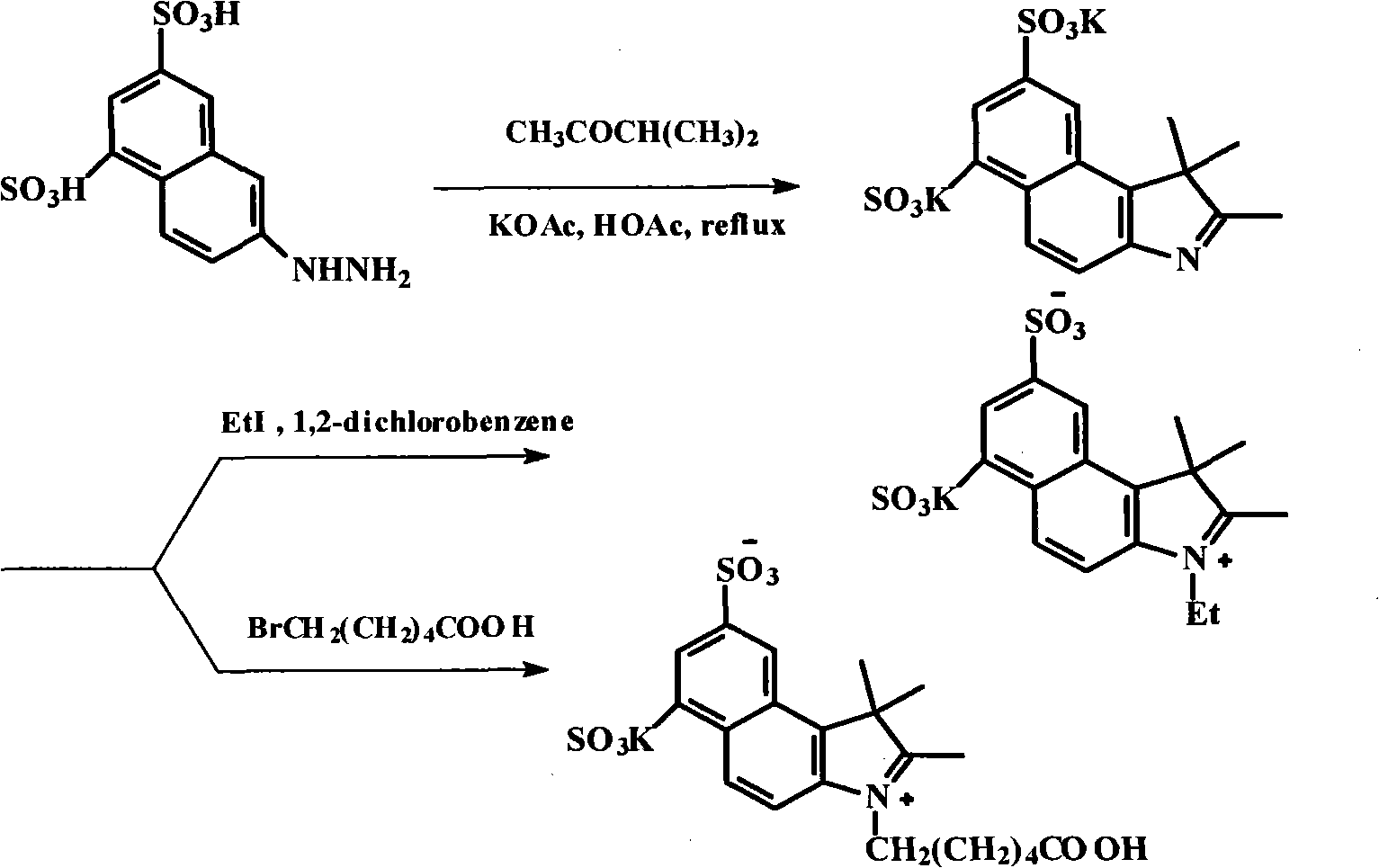
ActiveCN102010614AEasy to operateLow costMethine/polymethine dyesMethyl groupThin layer chromatogram
The invention discloses a method for preparing and purifying a water-soluble cyanine dye (Cy5.5), which comprises the following steps of: reacting 1,3-disulfonic acid-6-naphthylhydrazine serving as an initiative raw material with methyl isopropyl ketone to generate 1,1,2-trimethyl-benzindole-1,3-disulphonate; reacting the 1,1,2-trimethyl-benzo-indole-1,3-disulfonate with ethyl iodide and 6-bromocaproic acid respectively to generate N-ethyl-2,3,3-trimethyl-benzindole-5,7-disulphonate (product 1) and N-(epsilon-carboxy-pentyl)-2,3,3-trimethyl-benzindole-5,7-disulphonate (product 2); reacting the product 1 with beta-anilino-acraldehyde-anil-hydrochloride and then directly reacting the product 1 with the product 2 to prepare the Cy5.5; and preparing a thin layer chromatogram, and repeatedly purifying and separating the Cy5.5 to obtain a pure product of the Cy5.5. The method is easy and convenient to operate and has a high yield, the product does not need expensive high performance liquid chromatography (HPLC) (C-18 reverse column) for separation, and the method has great significance for meeting requirements of domestic autonomous production.
Owner:TIANJIN SUNGENE BIOTECH
Chinese medicinal composition and detection method for Chinese medicinal composition preparation
The invention relates to a detection method for a Chinese medicinal composition preparation, in particular to the detection method for a preparation for treating infantile common cold. The method carries out identification on thin-layer chromatographies of cablin potchouli herb and mint, and by repeated experimental observation, the chromatography of the tested preparation shows the spots with the same color and has suitable Rf value, good repeatability and no interference in the blank on a position corresponding to the chromatography of the reference medicaments. And the method also experiments on the method for measuring the content of patchouli alcohol in cablin potchouli herb, carries out optimization on experiment conditions, such as the temperature programming, the flow rate of carrier gas, the extracting time and the like, and repeatedly experiments on linear relation, precision, repeatability, stability and recovery ratio, and the result is excellent. The invention improves the controllability of the medicament quality and is beneficial for the quality control in the industrial production.
Owner:TONG REN TANG TECH CO LTD
Extraction process of ginseng saponin Rd
ActiveCN104610410APerfect purification stepsTrial production results showGlycoside steroidsN-ButanolSilica gel
The invention discloses an extraction process of ginseng saponin Rd. The extraction process comprises the following steps: S1, grinding ginseng saponin Rd-containing herbal materials into herbal powder, performing reflux extraction by using 70-95% ethanol, recycling the ethanol, and concentrating to obtain a total extract; S2, taking the total extract, enabling the total extract to pass through D101 macroporous adsorption resin, eluting by using 40-80% ethanol, recycling the ethanol, and performing deconperssion concentration to obtain total saponin; S3, taking the total saponin, enabling the total saponin to pass through a silica gel chromatography column, performing isocratic elution by using a mixture of n-butanol, ethyl acetate and water, performing thin layer chromatography, combining fractions to obtain crude ginseng saponin Rd; S4, taking the crude ginseng saponin Rd, enabling the crude ginseng saponin Rd to pass through an Rp-C18 silica gel column, performing isocratic elution by using a mixture of methanol and water, performing thin layer chromatography, combining required components, separating out precipitate to obtain the ginseng saponin Rd. By the extraction process, the ginseng saponin Rd separating and purifying effects can be improved, use of a toxic organic reagent is avoided, the operation is simple and convenient and a production process is easy to control; therefore, the extraction process is suitable for industrial production.
Owner:GUIZHOU XINBANG PHARMACEUTICAL CO LTD
Quality control method of Gongyankang grannule
The invention discloses a method for controlling the quality of the Gongyankang granules, revises and enlarges the thin layer chromatography (TLC) identification of bupleurum, semen plantaginis and baked ginger, purifies the method for extracting the sample of the content determination and the TLC identification of paeoniflorin which is a characteristic component of red paeony root, and revises and enlarges the method for examining the microorganism as well. The method for preparing the sample solution of the TLC identification of semen plantaginis and red paeony root in the quality control method is consistent with that of bupleurum, while the method for preparing the sample solution of the TLC identification of rhizoma corydalis is consistent with that of baked ginger, therefore, the quality control method has the advantages of more comprehensive detection items, higher resolution of content determination method, clearer TLC identification of red paeony root, more scientific and more reasonable quality control index, more simplified operation and further confirmed medicament curative effect.
Owner:广西厚德药业有限公司
Acidian polypeptide and preparation method thereof
The low-cost preparation method for polypeptide sea squirts with sequence as formula (I), 666.3 molecular weight and anti-HBV function comprises: dipping with ethanol at normal temperature to prepare extract after ethanol recovery; extracting the extract with organic solvent to condense, separate and purify by in turn the chromatography with macroporous resin, silica gel with gradient elution with CHCl3, MeOH and H2O by ratio as 7:3:0.5, and Sephadex LH20; collecting and condensing the eluent with Rf as 0.2-0.3 for ultraviolet detection label in TLC.
Owner:SOUTHERN MEDICAL UNIVERSITY
Discrimination method of traditional Chinese medicine Xuezhining pill
ActiveCN101653496AImprove detection abilityEasy to observe and identifyComponent separationMetabolism disorderSemenEthyl acetate
The invention relates to a discrimination method of traditional Chinese medicine Xuezhining pill; wherein discrimination of anthraquinones active ingredients in the Xuezhining pill and / or discrimination of nunciferine in Xuezhining pill all adopt thin-layer chromatography. Chrysophanol and physcion thin-layer chromatography in the discrimination method adopts acid hydrolysis method, thus greatly improving thin-layer spot detection effect, being convenient for observation and discrimination and improving discrimination efficiency; lotus leaf thin-layer chromatography in the discrimination method adopts alkalinity developing solvent cyclohexane-ethyl acetate-concentrated ammonium liquid, developing speed can be effectively improved, and discrimination speed of the whole Xuezhining pill can be improved. Repetitive operation of hawthorn thin-layer discrimination method is cancelled in the discrimination method, so that the whole quality control method is optimized, quality control time isshortened, and quality control efficiency is improved. The discrimination method of the invention is simple and easy to operate, eliminates the interference in discrimination as prepared polygonum multiflorum and semen cassiae all contain anthraquinones and is rapid and accurate for discrimination of traditional Chinese medicine Xuezhining pill.
Owner:津药达仁堂集团股份有限公司达仁堂制药厂
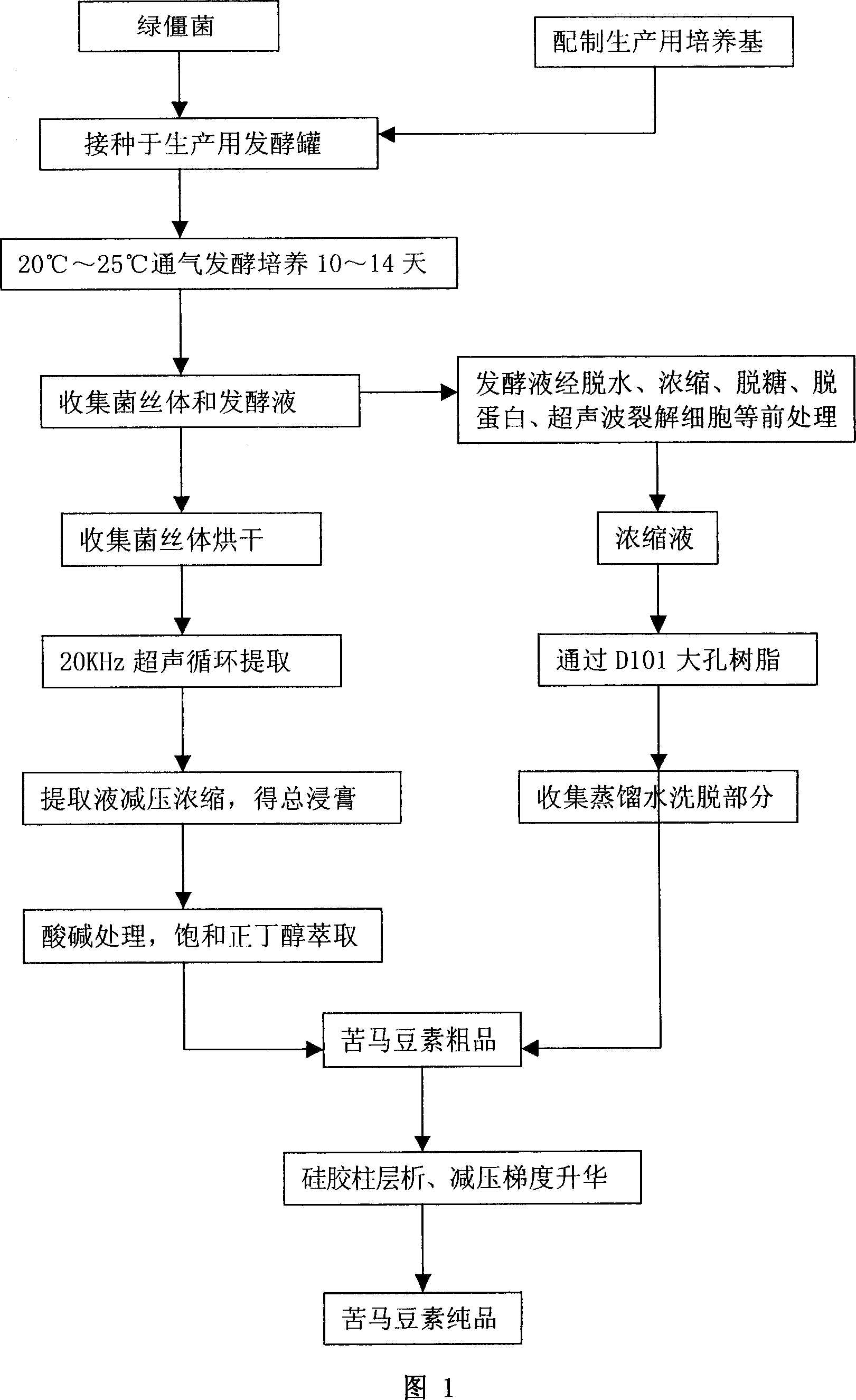
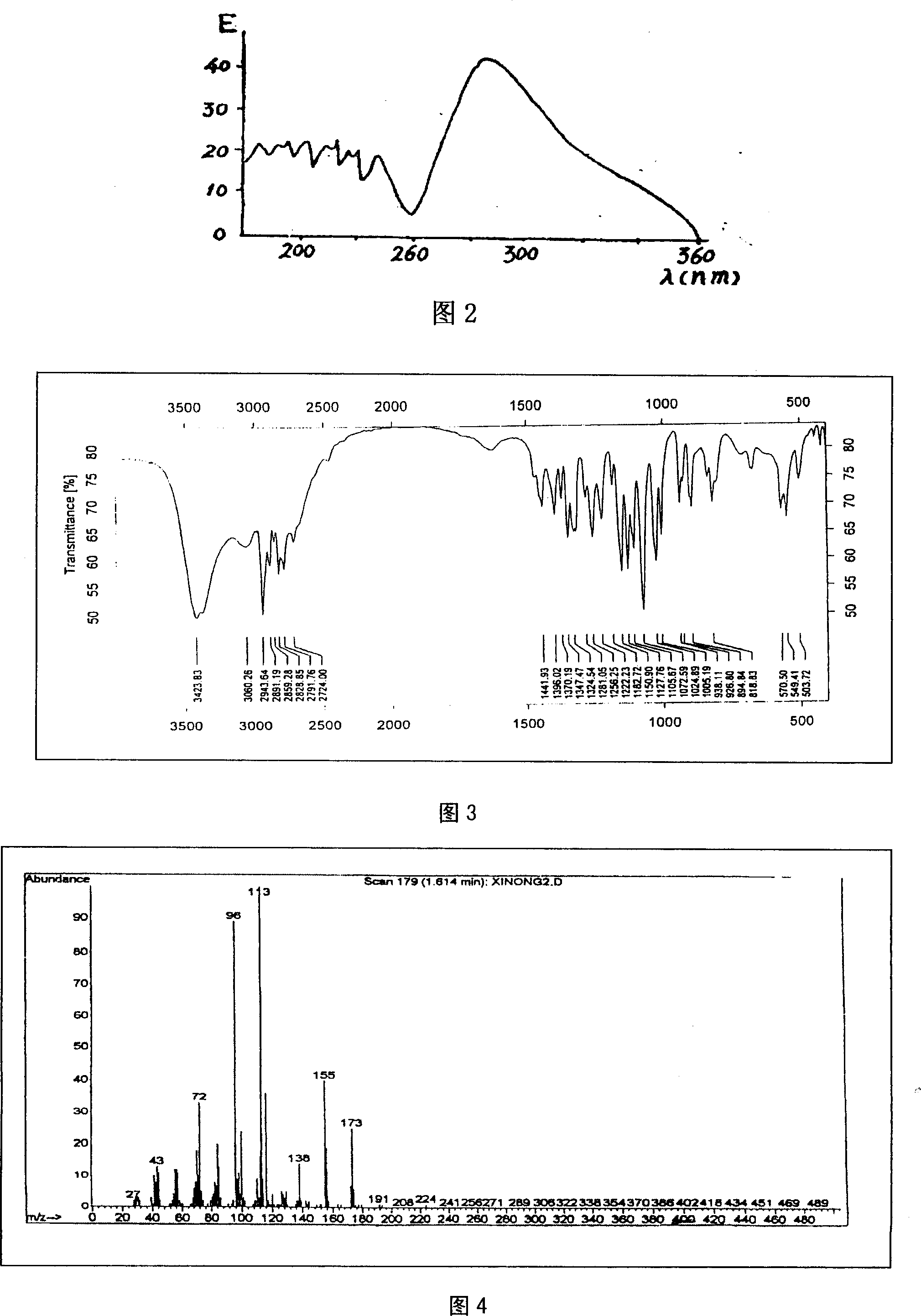
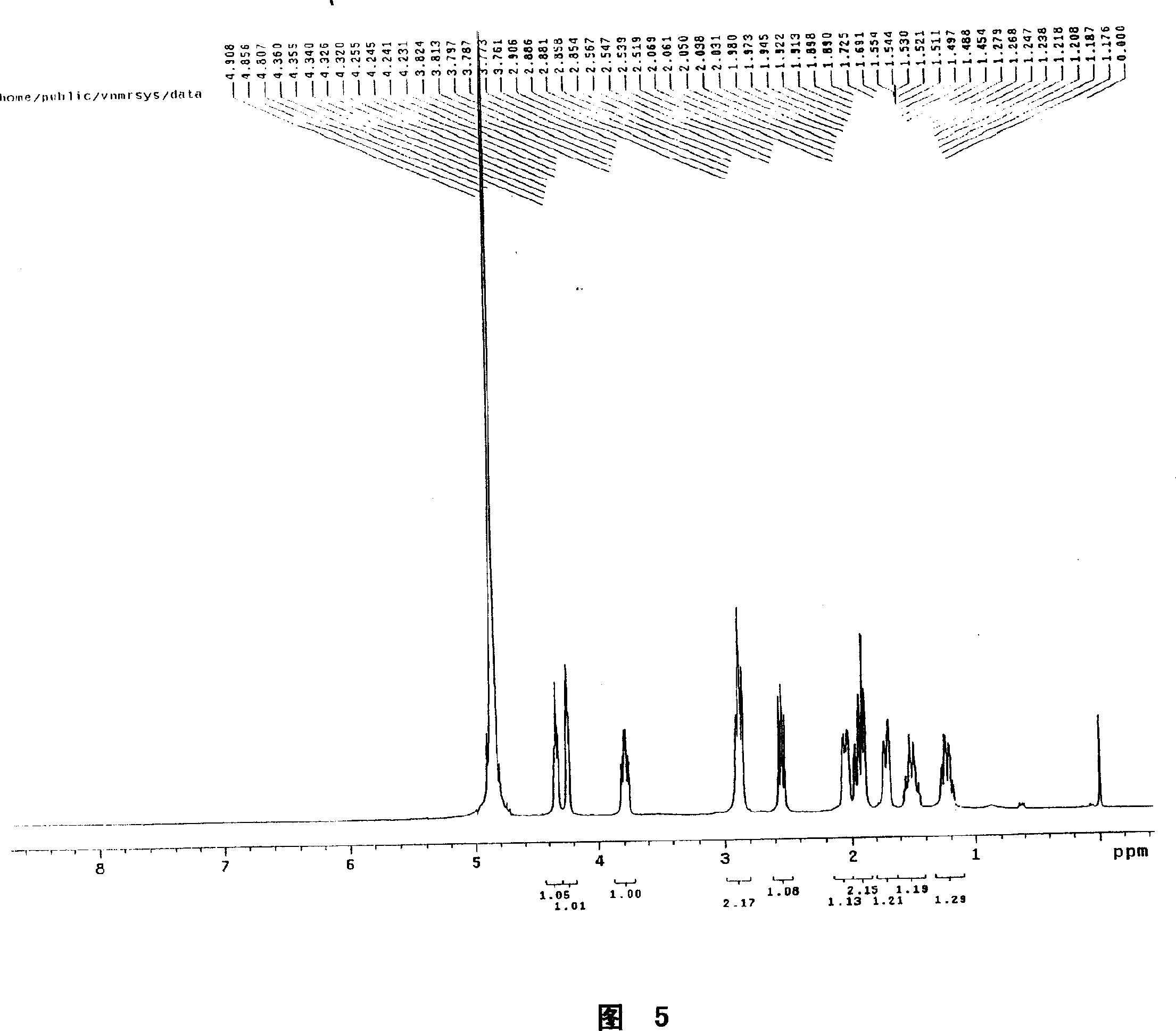
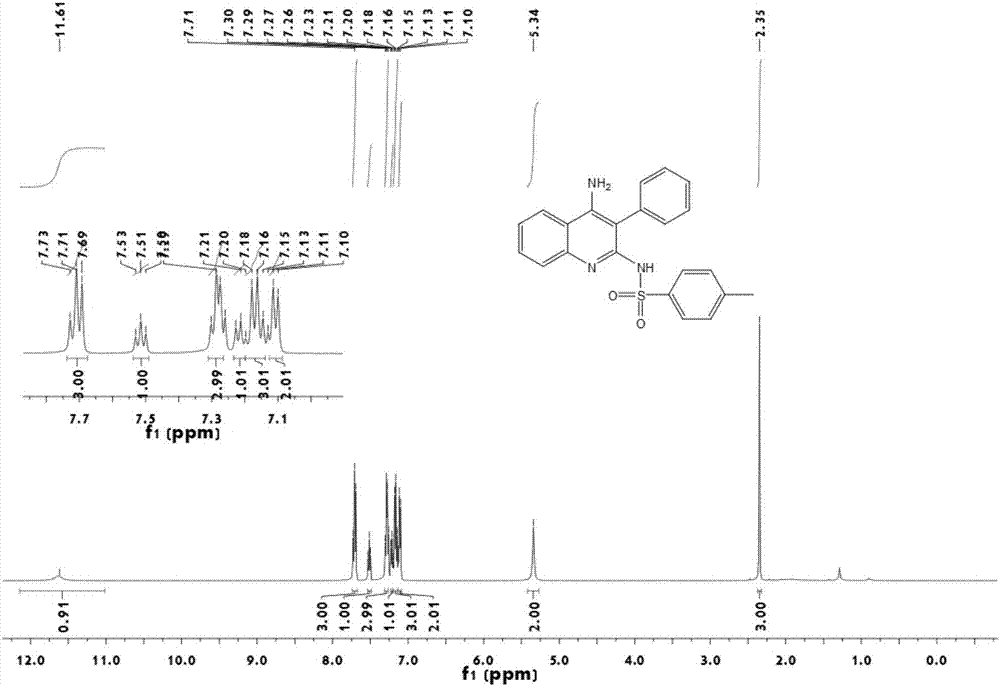
Technique for purifying spherosinin by fermenting green muscardine fungus
InactiveCN101100682AAddressing the ultimate scarcity problemConducive to ecological protectionMicroorganism based processesFermentationThin layer chromatogramCulture fungus
A process is concerned with purifying spherosin by fermentation of metarrhizzium anisopliae. It is carried out by: fermenting to obtain mycelium and fermented liquid, ultrasonic and solution extracting, and treating by thin-film chromatography, D101 resin, silica column chromatography and gradient sublimation to separate pure spherosin from mycelium and fermented liquid. The product is white needle crystal with molecular weight of 173, fusion point 144-145 deg.C, and purity being not less than 98%. The process does not damage present plant resources, and has short production period, costs low with mass production with high purity.
Owner:NORTHWEST A & F UNIV
Method used for preparing 4-aminoquinoline derivative
InactiveCN106866528ASignificant technological progressImprove reaction efficiencyOrganic chemistryEthylenediamineAlkyne
The invention discloses a method used for preparing a 4-aminoquinoline derivative. According to the method, an acuprous catalyst, an additive, and o-aminobenzontrile are delivered into a reaction container, wherein the additive is one ingredient selected from triethylamine, tetramethyl ethylenediamine, 1,8-diazabicyclo undec-7-ene, potassium carbonate, or cesium carbonate at will; an organic solvent is added; an alkyne-terminated compound and p-toluenesulfonyl azide are introduced into the reaction container; an obtained mixture is subjected to heating reaction for 3h to 6h at a temperature ranging from room temperature to 100 DEG C, wherein reaction process is monitoring via thin-layer chromatography, and optimal reaction conditions are obtained via selection; after reaction, an obtained mixture is filtered, obtained filter cake is washed, obtained filtrate is mixed, and is subjected to condensation and column chromatography separation so as to obtain the 4-aminoquinoline derivative. One-pot method is adopted to prepare the 4-aminoquinoline derivative, reaction efficiency is increased greatly, post-treatment is simple; and industrialized application prospect is promising.
Owner:SHANGHAI INST OF TECH
Detection method of Houttuynia herb injection
InactiveCN102323345AGood precisionGood repeatabilityComponent separationColor/spectral properties measurementsHouttuyniaThin layer chromatogram
The invention discloses a quality detection method of a Houttuynia herb injection. The method comprises content measurement, identification and / or fingerprint detection processes. The method is a gas chromatography, comprising the following steps of: taking a 4-terpineol reference substance, an alpha-terpinol reference substance, a bronyl acetate reference substance and methylnnonylketone, respectively adding n-hexane to prepare a reference substance solution; adding the n-hexane in the Houttuynia herb injection, adding anhydrous sulfate to prepare a test sample solution; respectively absorbing the reference substance solution and the test sample solution; injecting in the gas chromatography to test; and detecting according to the method of content measurement items, and recording a chromatogram, wherein the similarity of the test sample fingerprint and the reference fingerprint is not less than 0.85. The invention further provides a detection method of polysorbate 80 limit test in the Houttuynia herb injection, an identification method of thin-layer chromatography and a detection method of essential oil content in Houttuynia herb medical materials or injections. The quality detection method provided by the invention has good specificity, good linear relation, good instrument precision and good repeatability.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD +1
Preparation method of steroid intermediate
The invention discloses a preparation method of a steroid intermediate. The preparation method comprises the following steps: adding a first solvent, a first acid solution, water and 17-alpha-hydroxypregnenolone steroid-4,9-diene-3,20-diketone acetate into a first reaction tank, and uniformly stirring; adding a bromination agent in four times at 2-4 DEG C, maintaining the temperature for reactingfor 2h, and carrying out thin-layer chromatography analysis until the reaction of the raw materials is finished; and regulating the pH value to be more than 7, regulating the pH value back to 6, carrying out reduced pressure concentration until the first solvent is completely concentrated, adding water, cooling to 5 DEG C, and carrying out centrifugation, cleaning, spin-drying and drying, so as toobtain the steroid intermediate, namely cortisone. According to the preparation method, an oxidant and a catalyst which contain heavy metal ions are not used, the used solvent can be recycled and reused, wastewater does not contain heavy metal ions in the production process, the method belongs to an environment-friendly process and has very good process prospects, and the yield and purity of theproduct are high.
Owner:JIANGSU YUANDA XIANLE PHARMA
Quality control method of Ninggong tablet containing bezoar
InactiveCN101406674AGuaranteed quality and reliabilityGuaranteed quality uniformityHeavy metal active ingredientsNervous disorderQuality controlThin layer chromatogram
The invention discloses a quality control method for Niuhuang Ninggong tablet. The method comprises the following steps: morphological identification, histological and thin layer chromatography identification, arsenic trioxide detection, content measurement. The content of Baikal skullcap root in form of scutelloside in each tablet is no less than 0.8 mg, preferably no less than 1.0 mg, and most preferably no less than 1.1mg. The invention optimizes the prior examination method, establishes new examination method, ensures the reliability and uniformity of product quality and further improves the safety of products.
Owner:大连美罗中药厂有限公司
Compound preparation for treating bronchitis, its preparation method and quality control method
The invention discloses a process for preparing compound granule and the quality control method, wherein the compound granule is prepared from flitilarry bulb fluid extract, liquid extract of liquorice, platycodon glaucus, roor of sessile stemona, peucedanum root, pinellia tuber, dried orange peel, ammonium chloride, ephedrine hydrochloride and menthanol. The quality control method is characterized by the thin layer chromatogram authentication to dried orange peel, peucedanum root and licorice root, as well as the content measuring method for ephedrine hydrochloride.
Owner:张友生
Quality detection method for Liangfu pill-like preparations
ActiveCN105136966AEfficient separationImprove environmental adaptabilityComponent separationThin layerEthyl acetate
The invention discloses a quality detection method for Liangfu (of which the main compositions are rhizoma alpiniae officinarum and rhizoma cyperi) pill-like preparations. The method is a thin-layer chromatography method, and detection is performed by taking alpha-cyperone, cyperotundone, nootkatone, galangin and kaempferol as index components. The expansion conditions of the thin-layer chromatography comprise petroleum ether and ethyl acetate with the volume ratio of 5:1, and the thin-layer plate is silica gel GF254 plate. The quality detection method is capable of simultaneously detecting five index components in the Liangfu pill-like preparations, the environment adaptability is high, the method is simple, separation of the index components is good, and effective guarantee is provided for comprehensive detection on the quality of the Liangfu pill-like preparations.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Method for separating burdock oil, arctiin, arctigenin, lappaol E and lappaol H from burdock
ActiveCN105669797AHigh extraction rateHigh nutritional valueSugar derivativesOrganic chemistry methodsNutritive valuesGradient elution
The invention provides a method for separating burdock oil, arctiin, arctigenin, lappaol E and lappaol H from burdock. The method for separating the burdock oil, arctiin, arctigenin, lappaol E and lappaol H from the burdock comprises the following steps: firstly degreasing burdock through CO2 supercritical fluid extraction, separating to obtain the burdock oil and degreased burdock powder, carrying out water-alcohol extraction and concentration on the degreased burdock powder to obtain extract, carrying out CH2Cl2 extraction on the extract, loading the extract onto a silica gel column to be subjected to chromatography, carrying out dichloromethane-methanol gradient elution, carrying out thin-layer chromatography, and purifying, so as to obtain the arctiin, lappaol E, arctigenin and lappaol H. The method provided by the invention has the advantages that multiple products can be separated by adopting one continuous route only, raw materials are fully utilized, resources are saved, a degreasing process does not pollute the environment, the obtained burdock oil has high nutritive value, and the arctiin, arctigenin, lappaol E and lappaol H are high in purity and can be independently or mixed with other substances to be applied to the medicinal and edible field as raw materials.
Owner:CHONGQING KERUI PHARMA GRP
Method for controlling quality of Xingsu cough syrup
InactiveCN103175937AImprove quality control standardsAvoid product qualityComponent separationClinical efficacyBULK ACTIVE INGREDIENT
The invention provides a method for controlling the quality of Xingsu cough syrup. The method mainly comprises part or all of identification and content determination items, wherein the identification comprises thin-layer chromatography identification of perilla leaves, balloon flower, ephedra, thunberg fritillary bulb and baikal skullcap root; and the content determination means to determine the content of the main active ingredient amygdalin of bitter almonds in a preparation by adopting a high-performance liquid chromatography, and method validation is carried out, so that a reliable and accurate quality control method with strong specificity is established. By adopting the method, the quality control standard of the Xingsu cough syrup is improved, the quality of a product can be effectively controlled, and then the clinical curative effect of the product is definitely ensured.
Owner:蚌埠市第三人民医院
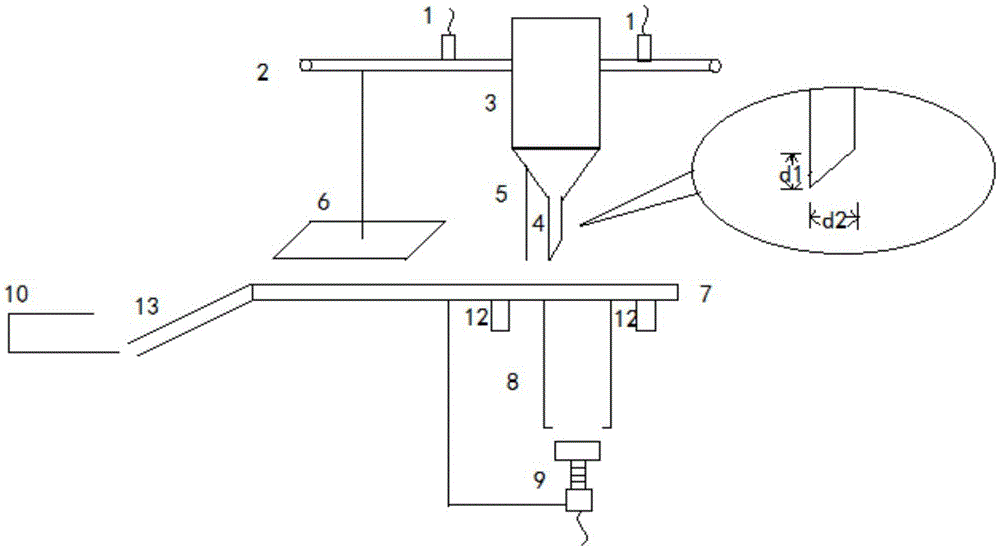
Fully-automatic thin-layer chromatography planking machine
ActiveCN105628852AReduce volumeImprove efficiencyComponent separationEngineeringUltrasonic vibration
The invention belongs to the technical field of thin layer chromatography preparation devices for chemical experiment and particularly relates to a fully-automatic thin-layer chromatography planking machine. The fully-automatic thin-layer chromatography planking machine is characterized in that a material mixing tank is positioned between two contact sensors and can move leftwards and rightwards on a supporting rod; the material mixing tank adopts a double-layer stainless steel plate, and an ultrasonic uniform mixing system is placed in an interlayer; materials are in full contact by using an ultrasonic vibration method; an automatic spiral column is adopted for regulating and controlling the planking thickness; a discharging port is used for smearing, a scraping plate is used for scraping and moving a thin layer chromatography plate, and a drying plate is used for drying. The fully-automatic thin-layer chromatography planking machine is small in size, high in efficiency and strong in practicability, homogenizes at a high speed, is free from dust and air bubbles and realizes planking integration and intellectualization.
Owner:DALIAN UNIV
Method for synthesizing drospirenone
The invention relates to a method for synthesizing drospirenone and belongs to the field of pharmaceutical chemicals, which comprises: reacting a 3beta,5beta-dyhydroxy-6beta,7beta,15beta,16beta-imethylene-17alpha-(3'-hydroxypropyl)-androstene-17ol compound serving as a raw material in dichloromethane in the presence of dichlorodimethylhydantoin, potassium bicarbonate and crown ether, which serve as catalysts, to obtain an 3-oxo-5beta-hydroxy-6beta, 7beta,15beta,16beta-dimethylene-17alpha(spiro)butyrolactone intermediate; removing excessive oxidant by using a small amount of sodium sulfite, filtering the solution, adding a certain amount of phosphorus pentoxide into solution of dichloromethane for dehydration, adding water for washing the reaction product for one time at the end ( detected by thin-layer chromatography) of the reaction and washing the reaction product for one time with saturated solution of sodium bicarbonate; drying the reaction product with anhydrous sodium sulfate, filtering the reaction product, distilling and recovering solvent and crystallizing the solid with water solution (in a volume ratio of 3:1) of methanol; and finally, recrystallizing the obtained solid with isopropylacetate to obtain a qualified drospirenone product. The synthesis yield of the method is about 67 percent. The reaction is mild and easy to operate and consumes a small amount of organic solvent.
Owner:HANGZHOU LONGSHAN CHEM CO LTD
Thin layer chromatography residue applicator sampler
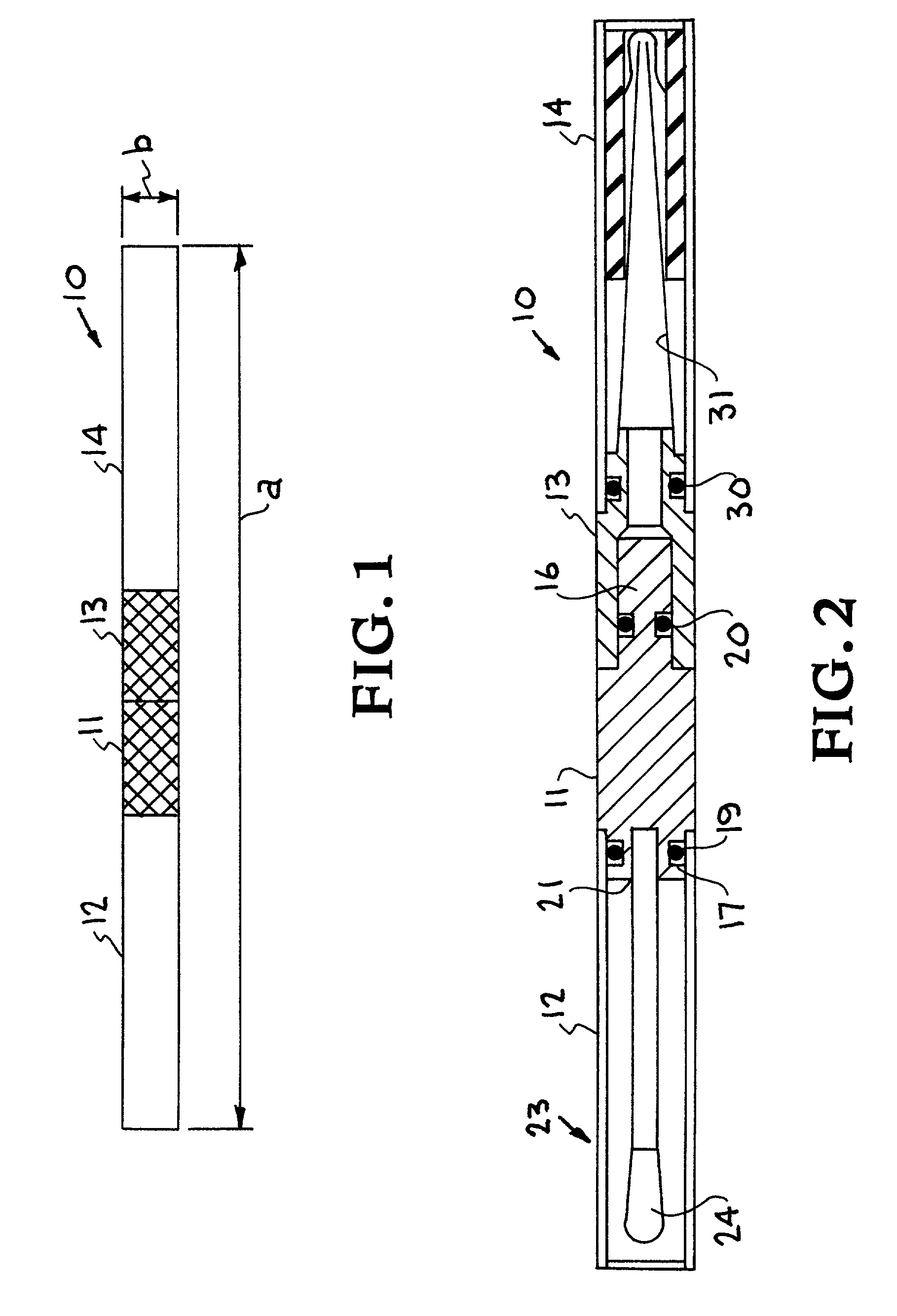
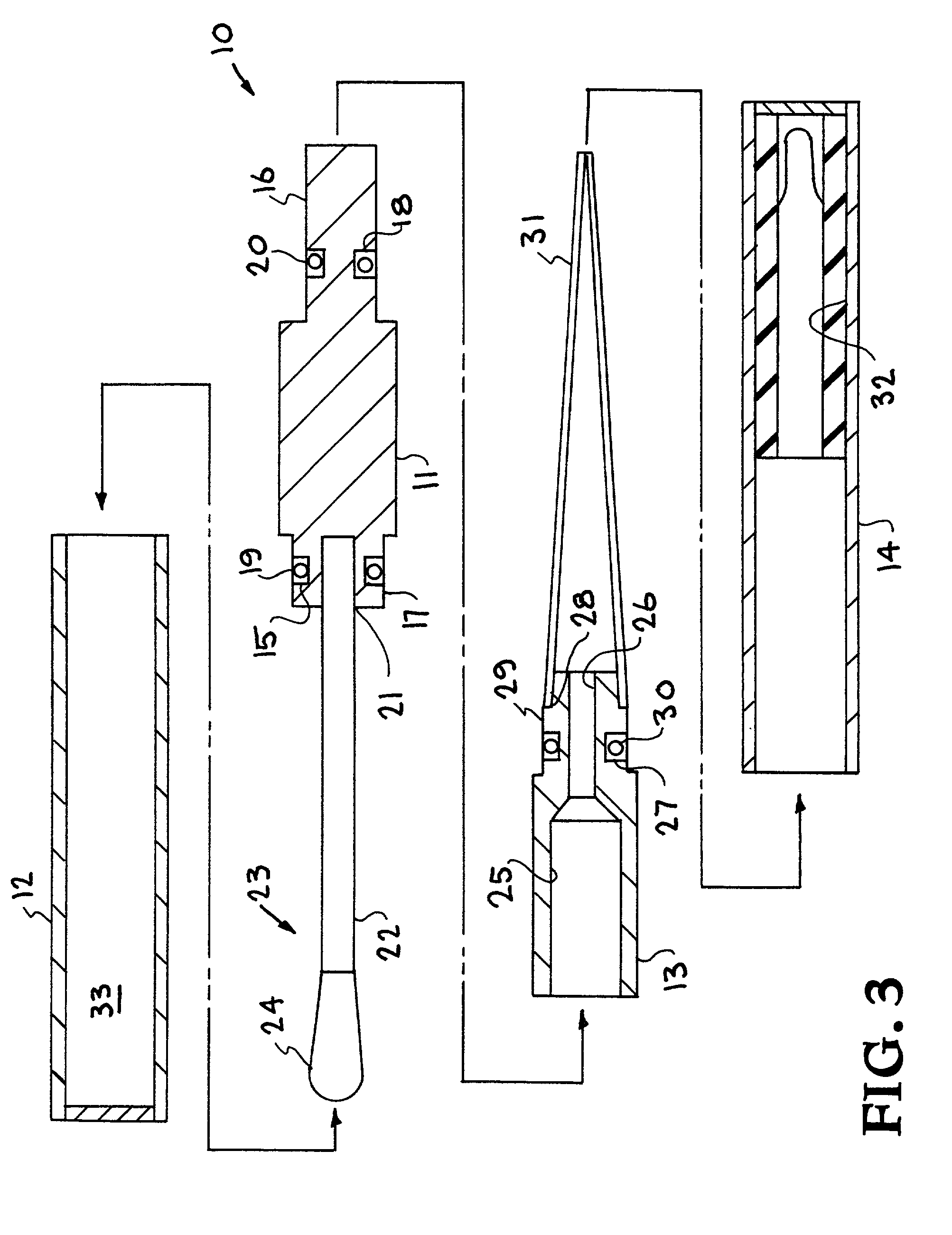
InactiveUS7247273B2Prevent leakageAvoid cross-contaminationComponent separationWithdrawing sample devicesHand heldDissolution
A thin layer chromatograph residue applicator sampler. The residue applicator sampler provides for rapid analysis of samples containing high explosives, chemical warfare, and other analyses of interest under field conditions. This satisfied the need for a field-deployable, small, hand-held, all-in-one device for efficient sampling, sample dissolution, and sample application to an analytical technique. The residue applicator sampler includes a sampling sponge that is resistant to most chemicals and is fastened via a plastic handle in a hermetically sealed tube containing a known amount of solvent. Upon use, the wetted sponge is removed from the sealed tube and used as a swiping device across an environmental sample. The sponge is then replaced in the hermetically sealed tube where the sample remains contained and dissolved in the solvent. A small pipette tip is removably contained in the hermetically sealed tube. The sponge is removed and placed into the pipette tip where a squeezing-out of the dissolved sample from the sponge into the pipette tip results in a droplet captured in a vial for later instrumental analysis, or applied directly to a thin layer chromatography plate for immediate analysis.
Owner:LAWRENCE LIVERMORE NAT SECURITY LLC
Method for detecting quality of lung clearing and phlegm eliminating pill
ActiveCN101732552AImprove quality control standardsHigh level of quality standardsComponent separationPill deliveryPublic healthMedicine
The invention relates to a method for detecting quality of a lung clearing and phlegm eliminating pill in Volume 12 of Drug Standard of Ministry of Public Health of Peoples Republic of China (traditional medicine prescribed preparation). The method for detecting quality of a lung clearing and phlegm eliminating pill comprises microscopic identification, a thin layer chromatography identification method for orange peel and bitter orange, a thin layer chromatography identification method for ephedrine hydrochloride in herba ephedrae, a thin layer chromatography qualitative identification method for balloon flower, and scutelloside content measurement. The invention enhances the quality control standard of the lung clearing and phlegm eliminating pill, establishes content measuring and detecting indexes in main medicines of preparation and a detecting method thereof, increases the thin layer chromatography qualitative identification methods for orange peel, bitter orange, ephedrine hydrochloride and balloon flower, and ensures the higher quality standard levels of the compound preparation.
Owner:KUNMING CHINESE MEDICINE FACTORY
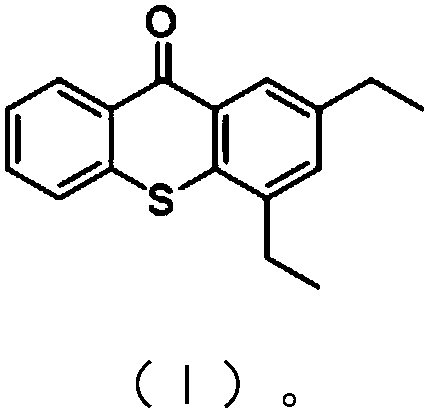
Preparation method of 2,4-diethylthioxanthone
The invention provides a preparation method of 2,4-diethylthioxanthone. By optimally using a solvent which has good solubility to a substrate, the reaction of 2,4-diethylbenzene sulfur phenolate and phthalonitrile is accelerated under the condition of homogeneous phase, and the reaction period is shortened by 70 percent or above when being compared with that of a traditional process; the cyclization reaction has mild conditions, the use amount of concentrated sulfuric acid in the reaction is reduced by about 80 percent compared with that of a traditional process, and the discharge of waste acid and waste water is greatly compressed, so that the aims of energy saving and emission reduction are achieved. The two-stage reaction can be accurately controlled by thin-layer chromatography, the operation is simple and convenient, the post process is convenient, and the total yield reaches 85 percent or above; the preparation method of the 2,4-diethylthioxanthone is suitable for industrializedproduction.
Owner:TIANJIN RUILING CHEM CO LTD
Quality control method for Zengguang capsules
The invention provides a new quality control method for Zengguang capsules and overcomes the drawbacks of the prior art. In the invention, the content of ferulic acid in the compound preparation, namely the Zengguang capsules, is measured by high efficiency liquid chromatography; and the thin-layer chromatography identification of medlar, which is the main medicine of the compound preparation, and medicinal materials including Codonopsis pilosula and Schisandra chinensis is performed, so the accuracy and advancement of the quality control method of the compound preparation are ensured, and the product quality of the Zengguang capsules can be controlled more effectively.
Owner:ZHENGZHOU FURUITANG PHARMA
Preparation method and use of hemp seed reference extract product
InactiveCN104398580AReduced stabilityImprove stabilityComponent separationPlant ingredientsWater bathsFiltration
preparation method of a hemp seed reference extract product comprises the following steps: 1, carrying out ether degreasing: taking 200g of hemp seed medicinal material powder, sieving by a No.2 sieve, adding 1500ml of ether, carrying out water bath heating refluxing for 1h at a water bath temperature of 50DEG C, carrying out reduced pressure filtration, adding 500ml to medicinal residues, washing, and removing the obtained ether liquid; 2, extracting with methanol to obtain an extract: adding 1500ml to the obtained medicinal residues, carrying out water bath heating refluxing at 70DEG C for 1h for extraction, carrying out reduced pressure filtration, and evaporating to remove methanol to obtain an extract, and weighing; and 3, carrying out silicon gel mixing and reduced pressure drying: re-dissolving the extract obtained in step 2 in 100ml of methanol, adding column chromatography silica gel having a same amount with the extract, uniformly mixing, carrying out reduced pressure evaporation to remove methanol, and sieving the obtained substance by a No.9 sieve in order to obtain the hemp seed reference extract product. The invention also provides a use of the hemp seed reference extract product. The hemp seed reference extract product substitutes a hemp seed reference medicinal material to be used as a reference for thin layer chromatogram discrimination.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Quality control method of xiaojiean preparation
ActiveCN102198210AAccurate identificationReliable identificationComponent separationAntineoplastic agentsMotherwortMedicine
The invention relates to a quality control method of a medicine preparation, and especially relates to thin layer chromatogram authentication and content determination of smilax glabra in a xiaojiean preparation. The method is a thin layer chromatogram authentication and / or content determination method formulated by using a special component in the smilax glabra of the xiaojiean preparation as an index component, wherein the special component is astilbin, and the xiaojiean preparation is a traditional Chinese medicine compound preparation prepared from 1100 parts of leatherleaf mahonia, 750 parts of thin evodia, 750 parts of motherwort, 750 parts of spatholobus stem, 900 parts of smilax glabra and 750 parts of fructus forsythiae. The thin layer chromatogram authentication method with a strong specialization can authenticate smilax glabra accurately and reliably, and a generally employed HPLC can determine the astilbin content in the xiaojiean preparation, so as to produce valuable significance for monitoring and controlling medicinal material purchase, preparation production process and preparation quality in market, and ensuring product safety, effectiveness and quality stabilization. According to the method, problem of confused smilax glabra basic material provided in the market can be solved effectively to ensure that xiaojiean preparation meets the national medicine standards strictly.
Owner:云南神威施普瑞药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com