Method for rapidly and simultaneously detecting ten adulterated components in lipid lowering Chinese patent medicine
A technology for Chinese patent medicines and lipids, which is applied in the detection field of lipid-lowering Chinese patent medicines, can solve the problems of missed detection and non-qualitative detection, and achieve the effects of simple operation, high sensitivity and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] (1) Preparation of nano-silver colloid solution: Accurately weigh 8.5mg of PVP (polyvinylpyrrolidone) and 17mg of silver nitrate, dissolve them in 5mL of water, and obtain AgNO with a mass ratio of 2:1. 3 / PVP mixed solution. Measure 50mL of DMF (N,N-dimethylformamide) into a 250mL three-necked bottle, and heat to slightly boiling. Quickly add AgNO as above 3 / PVP solution and continue to boil for a period of time, naturally cool to room temperature and store in a brown bottle away from light and low temperature.
[0042](2) Preparation of a single adulterated component standard solution: Precisely weigh 1 mg of the reference substance and dissolve it in 1 mL of methanol (analytical grade), and use ultrasonic treatment for 30 to 50 minutes to obtain ten adulterated components with a concentration of 1 mg / mL. standard solution.
[0043] (3) Preparation of the matrix solution of lipid-lowering Chinese patent medicine samples: 10 mg of lipid-lowering Chinese patent medi...
Embodiment 1
[0048] In this Example 1, assuming that fenofibrate is contained in lipid-lowering Chinese patent medicines, the method for quickly and simultaneously detecting ten adulterated ingredients in lipid-lowering Chinese patent medicines is explained in detail. The steps are as follows:
[0049] Step 1, preparing matrix solution, reference substance solution (fenofibrate standard solution) and simulated positive sample solution, prepared according to the above method;
[0050] Step 2, determination of surface-enhanced Raman spectrum of fenofibrate standard
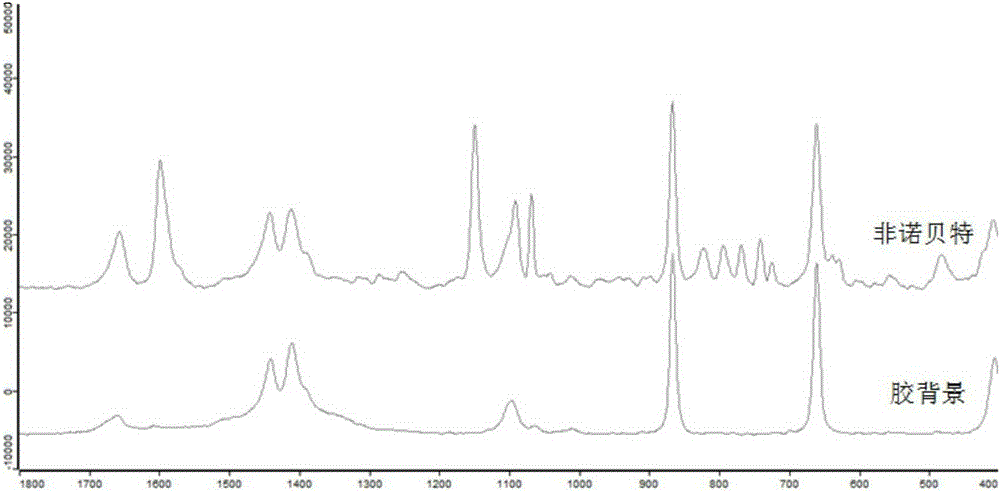
[0051] Spot the fenofibrate standard solution of 1mg / mL on the thin-layer plate to obtain spots, and then drop 5uL nano-silver colloid solution on the spots, and use a portable Raman spectrometer to detect the spots on the thin-layer plate. Laser power 300mw, integration time 15s, obtain the surface-enhanced Raman spectrum of this reference substance, specific as figure 2 shown.
[0052] Step 3: Determine the TLC plate develo...
Embodiment 2
[0058] In this second example, assuming that bezafibrate is contained in lipid-lowering Chinese patent medicines, the method for rapid and simultaneous detection of ten adulterated ingredients in lipid-lowering Chinese patent medicines is explained in detail. The steps are as follows:
[0059] Step 1, preparing matrix solution, reference substance solution (bezafibrate standard solution) and simulated positive sample solution, prepared according to the above method;
[0060] Step 2, determination of the surface-enhanced Raman spectrum of the bezafibrate standard
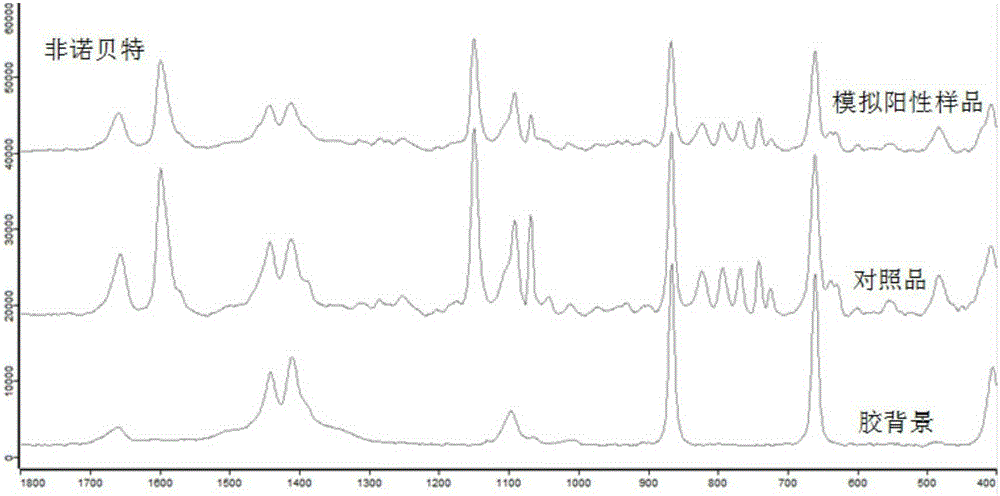
[0061] Spot the fenofibrate standard solution of 1mg / mL on the thin-layer plate to obtain spots, and then drop 5uL nano-silver colloid solution on the spots, and use a portable Raman spectrometer to detect the spots on the thin-layer plate. Laser power 300mw, integration time 15s, obtain the surface-enhanced Raman spectrum of this reference substance, specific as Figure 5 shown.
[0062] Step 3: Determine the TLC ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com