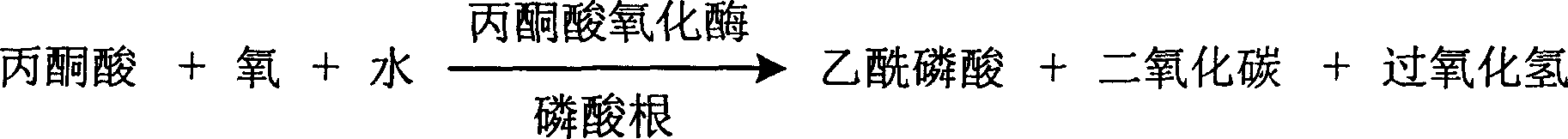Patents
Literature
69results about How to "Determination of content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for detecting contents of glyceride and free fatty acid in biodiesel
ActiveCN104237447AEfficient detectionDetermination of contentComponent separationBiodieselMonoglyceride
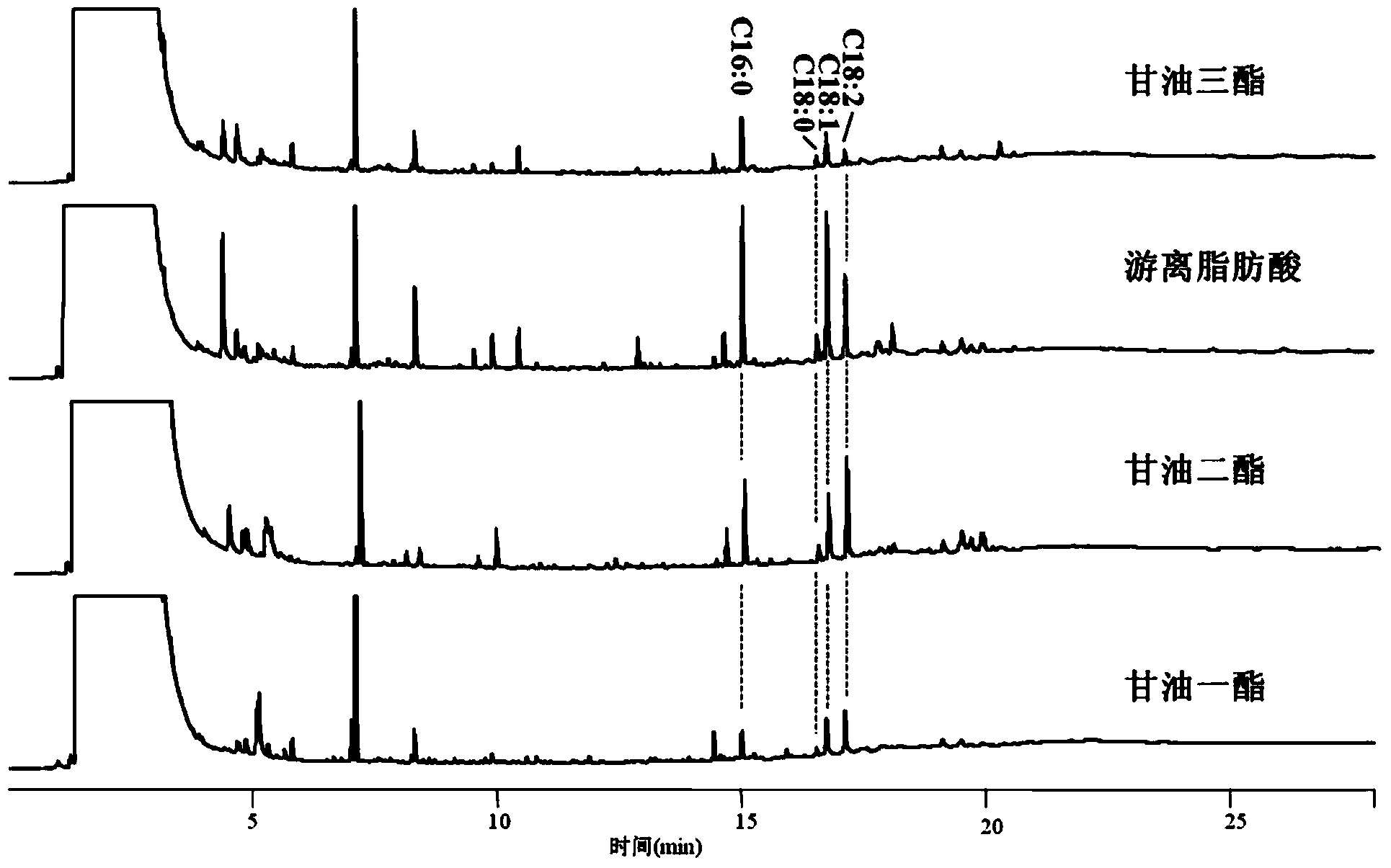
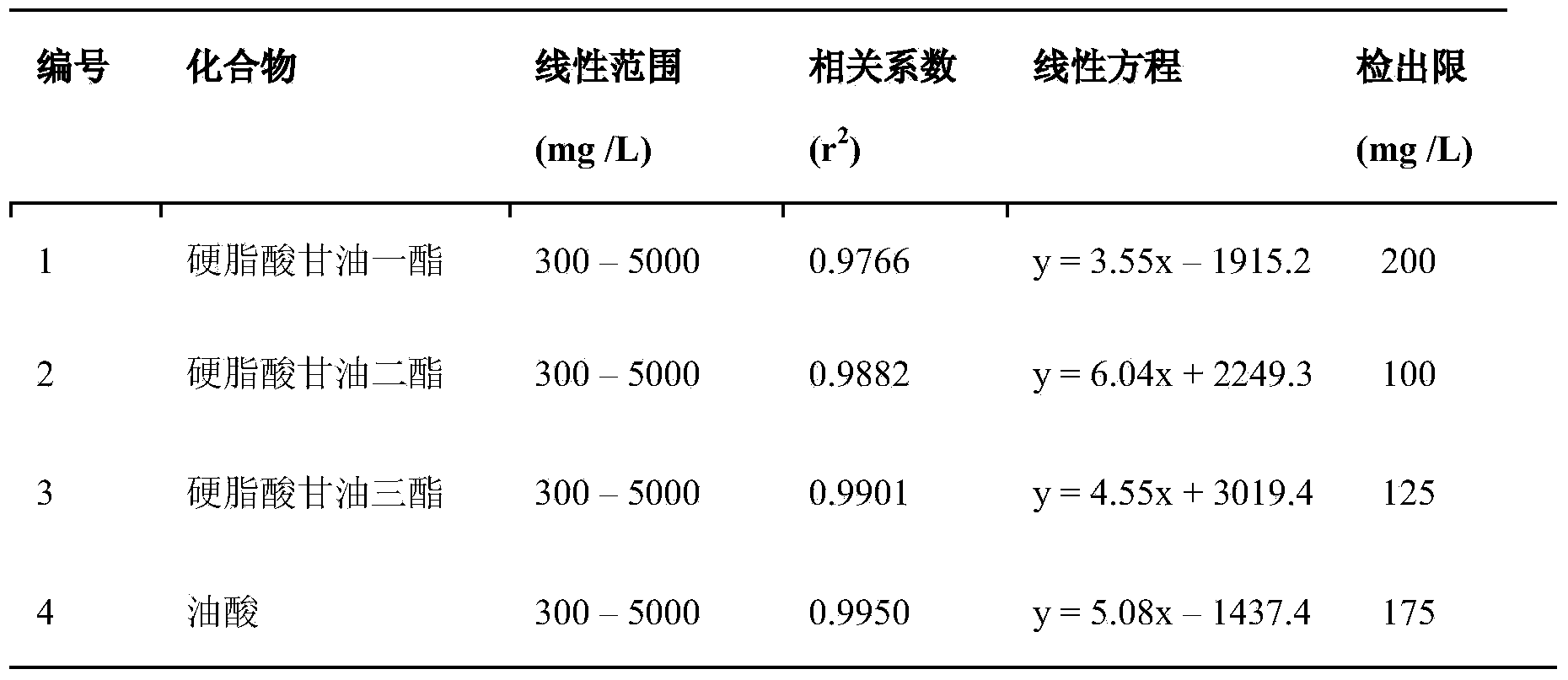
The invention discloses a method for detecting the contents of glyceride and free fatty acid in biodiesel. The method comprises the following steps: diluting a biodiesel sample, carrying out thin layer chromatograph with an aluminum matrix silica gel plate; with n-hexane, acetic ether and formic acid mixed solvent as a developer, developing with iodine steam, wherein the volume ratio of the n-hexane, acetic ether and formic acid is 90:10:2; after separating, cutting off the spots of monoglyceride, diglyceride, glycerin trilaurate and free fatty acid; putting the cut spots and carbinol solution of three methyl hydrogen trioxide into a sample cup; putting the sample in a cracker; placing the cracker at a GC sample feeding opening, and feeding the sample when the temperature of the cracker reaches 300-450 DEG C; carrying out gas chromatograph detection to obtain the gas chromatogram of the to-be-detected sample; comparing the gas chromatogram with the standard curves of monoglyceride, diglyceride, glycerin trilaurate and free fatty acid and calculating to obtain the contents of glyceride and free fatty acid in the to-be-detected sample. The method is simple in operation, accurate in quantization and comprehensive in information and has profound significance on quality control of the biodiesel.
Owner:仕宝(天津)技术检测有限公司
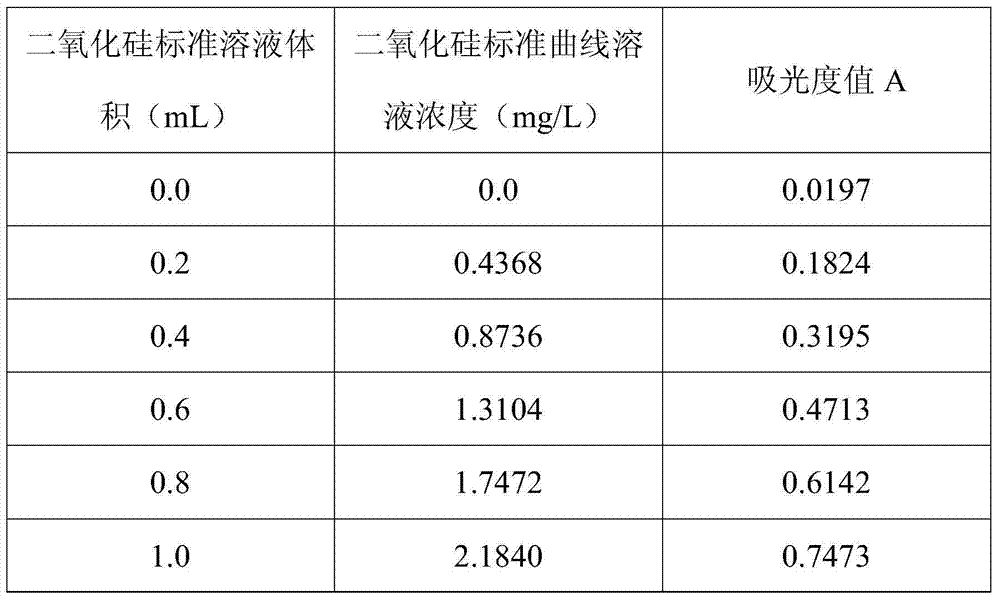
Method for determining content of silicon dioxide in rubber through spectrophotometer
InactiveCN103674868AWide measurement rangeSmall amount of sampleColor/spectral properties measurementsAmmonium ferrous sulfateMolybdenum blue
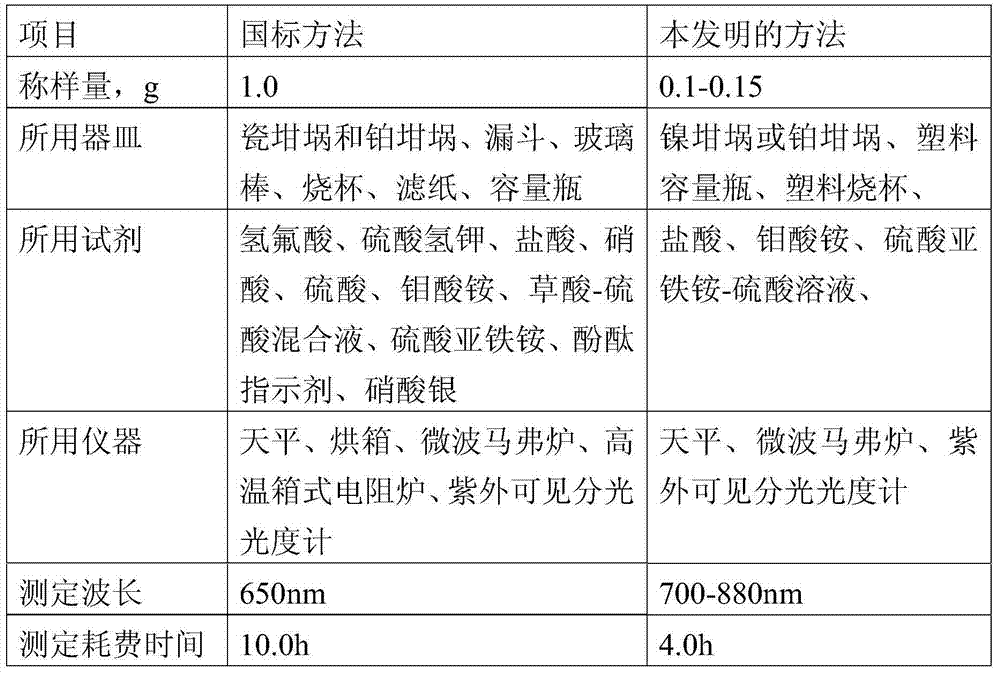
The invention relates to a method for determining the content of silicon dioxide in rubber through a spectrophotometer. The method comprises the steps as follows: subjecting a rubber sample to ashing treatment; adding potassium hydroxide into the ashed sample for treatment so as to convert silicon in the sample into soluble silicate; then reacting a silicon-containing solution with ammonium molybdate in a hydrochloric acid medium; then using ammonium ferrous sulfate for reducing a reaction product in the last step to silicon molybdenum blue, and measuring the absorbance of the silicon molybdenum blue in a position with a set wavelength of 700-880 nm. The method is applied to all rubber except silicon-containing rubber.
Owner:BEIJING RED AVENUE INNOVA
Method for simultaneous determination of six active components in Niuhuang Ninggong tablet
InactiveCN104897787ASimple methodAccurate methodComponent separationPhosphoric acidColumn temperature
The invention discloses a method for simultaneous determination of six active components consisting of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride in a Niuhuang Ninggong tablet through HPLC. Chromatographic conditions employed in the invention are as follows: a chromatographic column is TC-C18 (4.6 mm * 250 mm, 5 [mu]m); detection wavelength is 280 nm; a mobile phase is methanol-0.05% phosphoric acid; gradient elution comprises three parts, i.e., elution with methanol with a concentration varying in a range of 10 to 80% in the time period from 0 min to 35 min, then elution with methanol with a concentration of 80% in the time period from 35 to 50 min, and finally elution with methanol with a concentration varying in a range of 80 to 10% in the time period from 50 to 60 min; flow velocity is 1.0 mL / min; column temperature is 25 DEG C; and sample size is 10 [mu]L. Under the above-mentioned chromatographic conditions, chromatographic peaks are perfectly separated, and concentrations and peak areas of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride show good linear relation. The method is simple, rapid and accurate, has good repeatability and can provide quality bases for comprehensive evaluation and control of the Niuhuang Ninggong tablet.
Owner:JILIN NORMAL UNIV
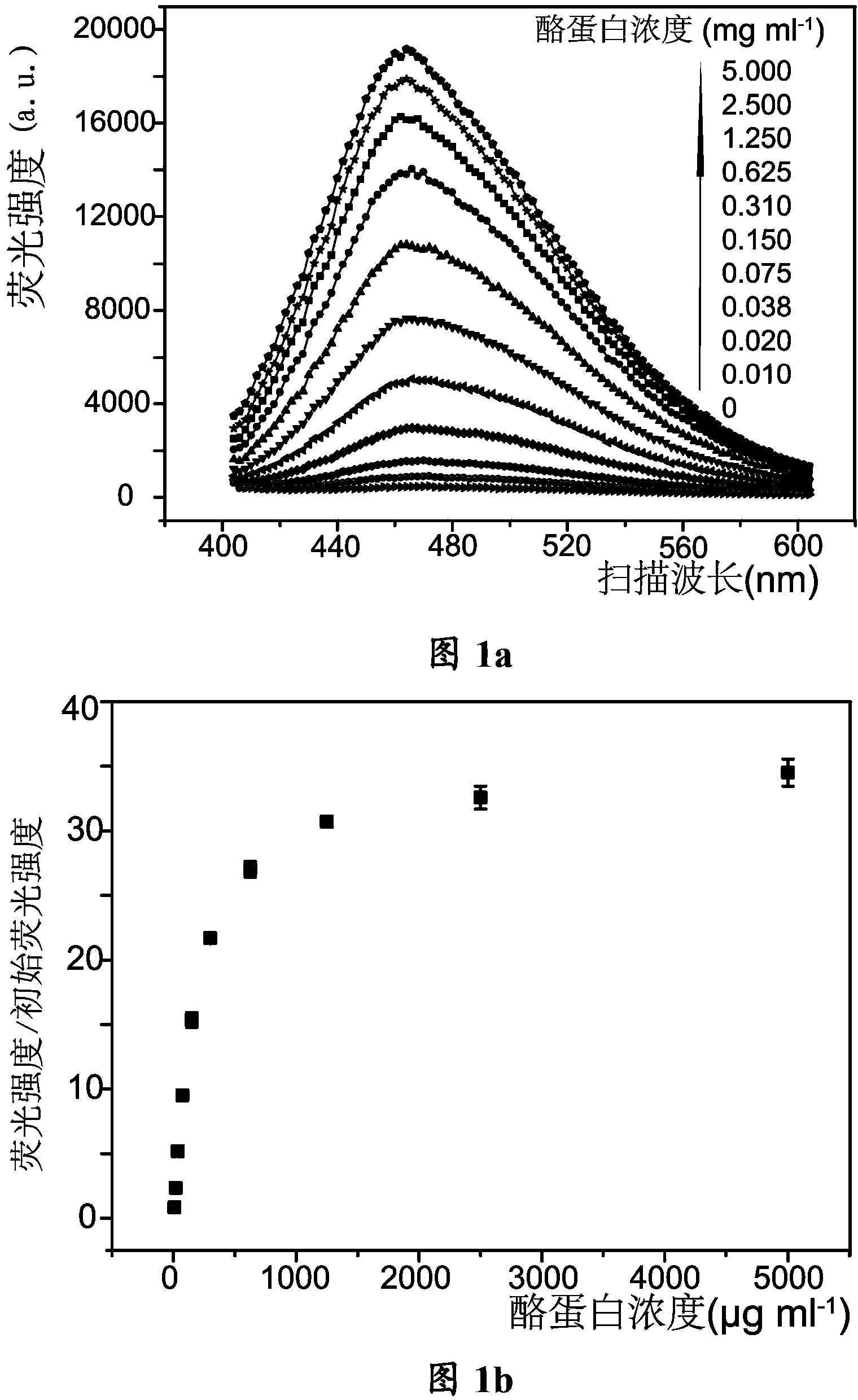
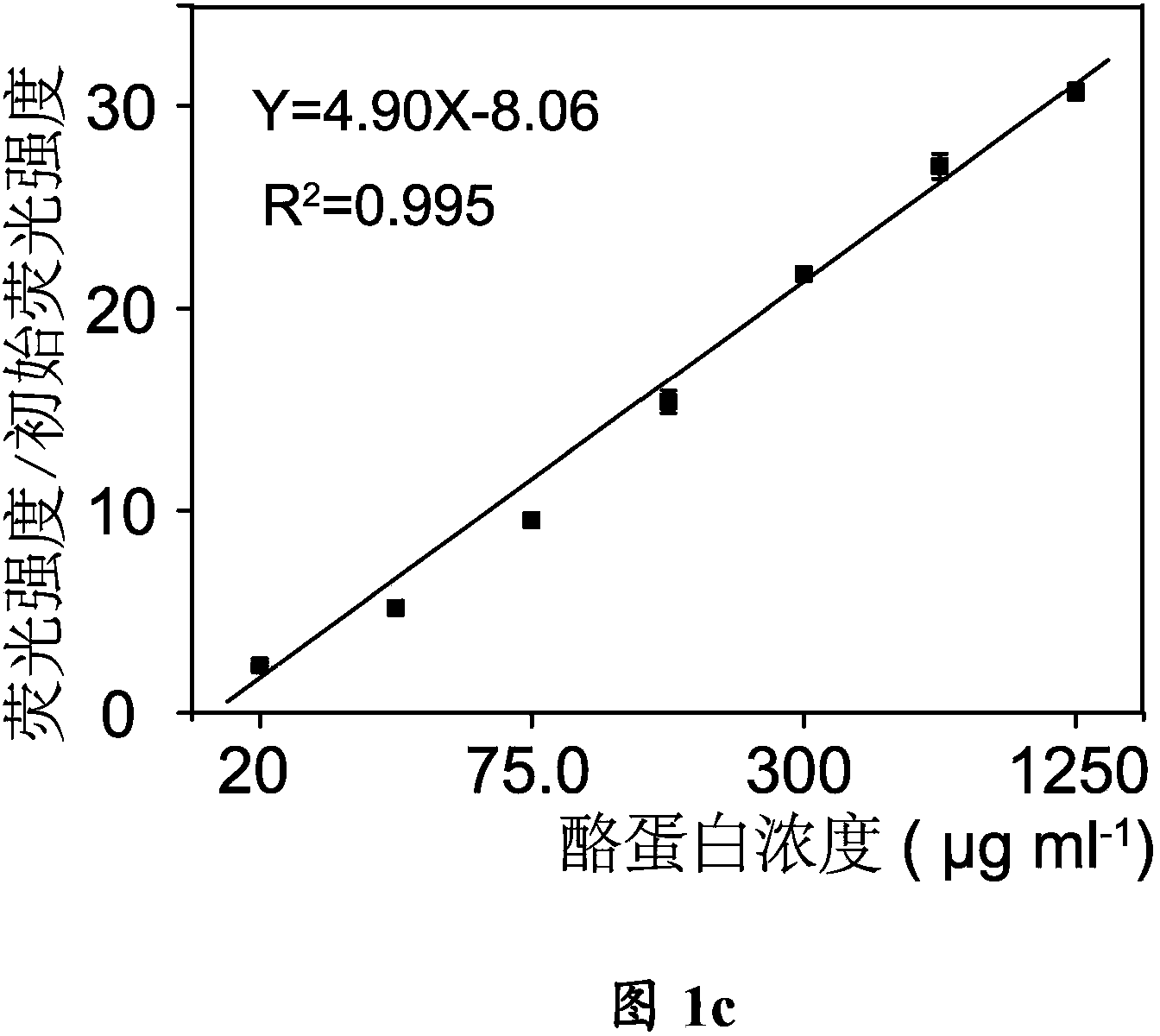
Method of detecting casein in dairy products by using tetraphenylethylene derivatives
InactiveCN103630529AEffectively measure contentDetermination of contentChemiluminescene/bioluminescenceProtein structureStructural formula
The invention provides a method of detecting casein in dairy products by using tetraphenylethylene derivatives, and particularly provides an application of the tetraphenylethylene derivatives in preparing products used for detecting the casein in the dairy products. The structural formula of the tetraphenylethylene derivatives is shown as the structural formula I. The method includes preparing a standard curve; precipitating the casein in the dairy products; dissolving the precipitated casein; mixing the dissolved casein with a solution of the tetraphenylethylene derivatives and measuring the fluorescence value; and comparing the measured fluorescence value with the standard curve to obtain the content of the casein. According to the method, the tetraphenylethylene derivatives capable of aggregation and luminescence are adopted. When encountering the casein in the dairy products, the tetraphenylethylene derivatives can enter into the protein structure to form an aggregation state and emit fluorescence to avoid interference, and therefore the content of the casein in the dairy products, such as milk and milk powder can be effectively measured. Formula (I).
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
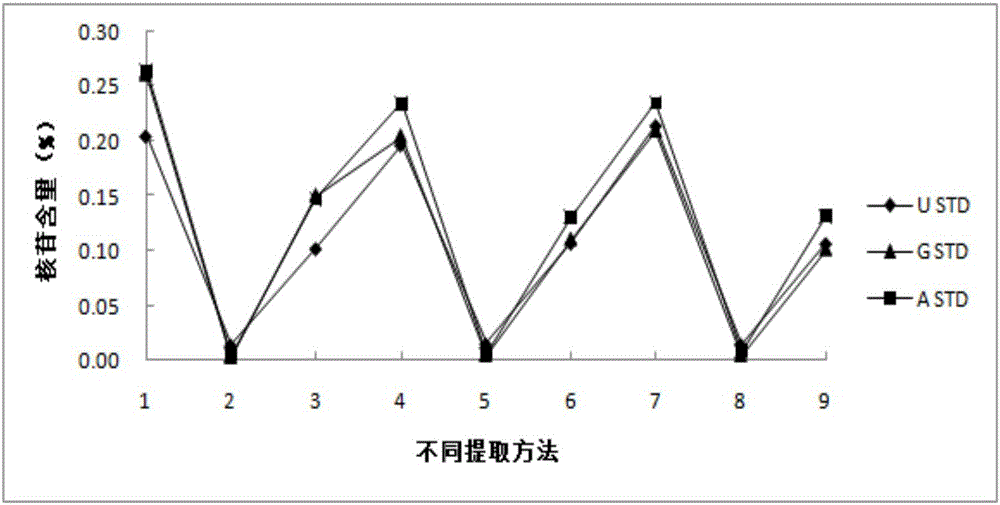
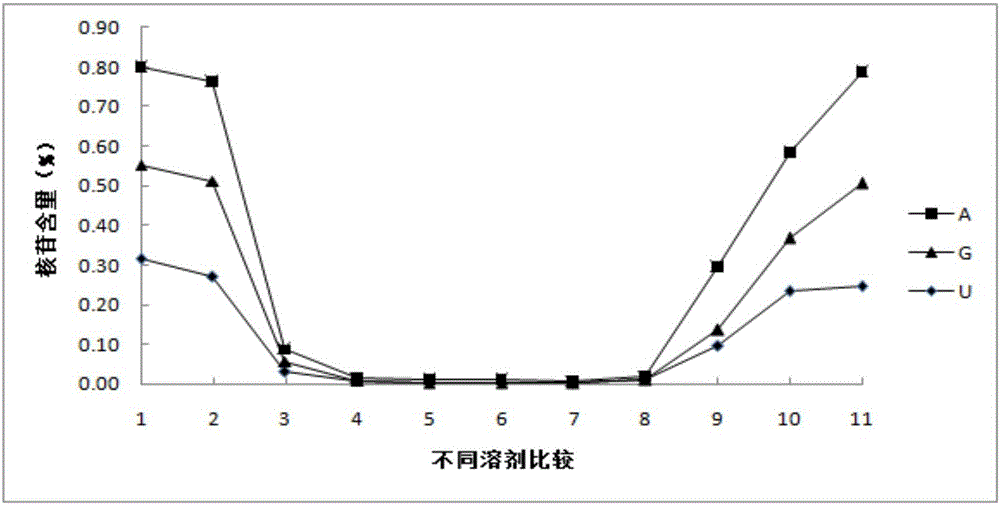
Method for detecting nucleoside components in Morchella esculenta
The invention belongs to the field of medical domestic fungus analysis and detection and relates to a method for simultaneous detection of guanosine, uridine and adenosine in Morchella esculenta by HPLC. The method comprises adding water into Morchella esculenta powder, carrying out constant temperature ultrasonic extraction, carrying out full shaking, taking the supernatant, filtering the supernatant through a water filter head, carrying out standing to obtain a sample solution to be detected, preparing a nucleoside reference solution and detecting nucleoside components in Morchella esculenta through HPLC. The method for detecting nucleoside components in Morchella esculenta has high stability, good repeatability and a good precision, produces a reliable and convenient result and can provide scientific basis for quality control of Morchella esculenta.
Owner:东莞东阳光保健品研发有限公司
Material sensitive to sulfide and hydrogen peroxide as well as preparation method and application thereof
InactiveCN101799418AHigh strengthDetermination of contentFluorescence/phosphorescenceHydrogenGas phase
The invention relates to a material sensitive to sulfide and hydrogen peroxide as well as a preparation method and application thereof in detecting sulphions and the hydrogen peroxide in a solution and sulfureted hydrogen in a gas phase, belonging to the field of composite materials. The material has a PbO / SiO2 structure, wherein the mole ratio of Pb and Si is 1:6.67-100. The material sensitive to the sulfide and the hydrogen peroxide can fast and conveniently measure a minute amount of the sulphions and the sulfureted hydrogen and can also carry out oxidation treatment on a specific oxidant and then repeatedly use the specific oxidant or use the specific oxidant to measure the hydrogen peroxide after the measurement, thereby being more economic; and in addition, the invention has the advantages of convenient use in measuring the sulphions and the sulfureted hydrogen, low cost, high sensitivity, fast response and very good market prospect.
Owner:SICHUAN UNIV
High performance liquid chromatography method for measuring content of hypocrellin A
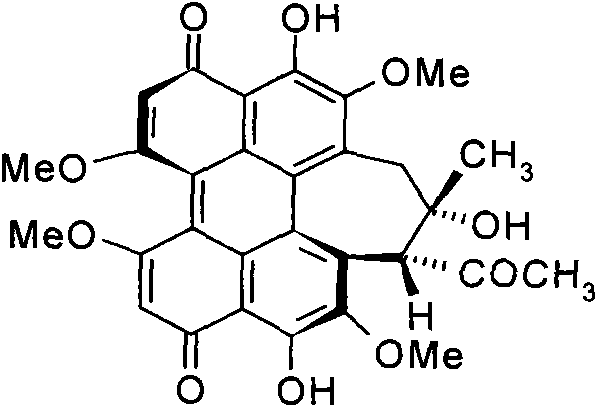
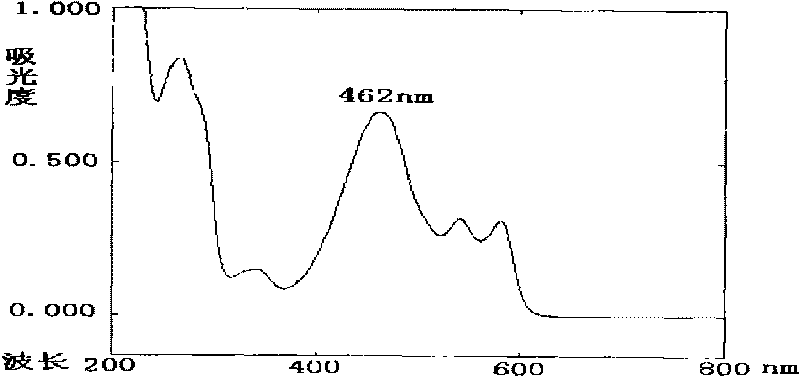
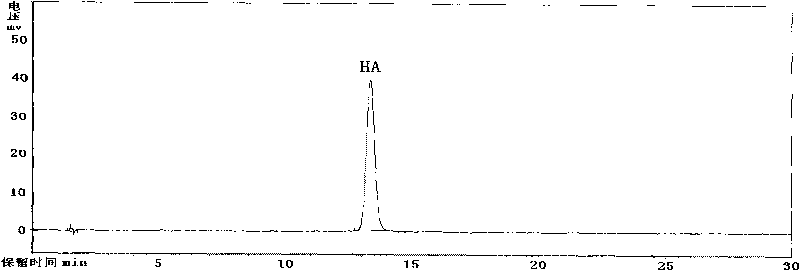
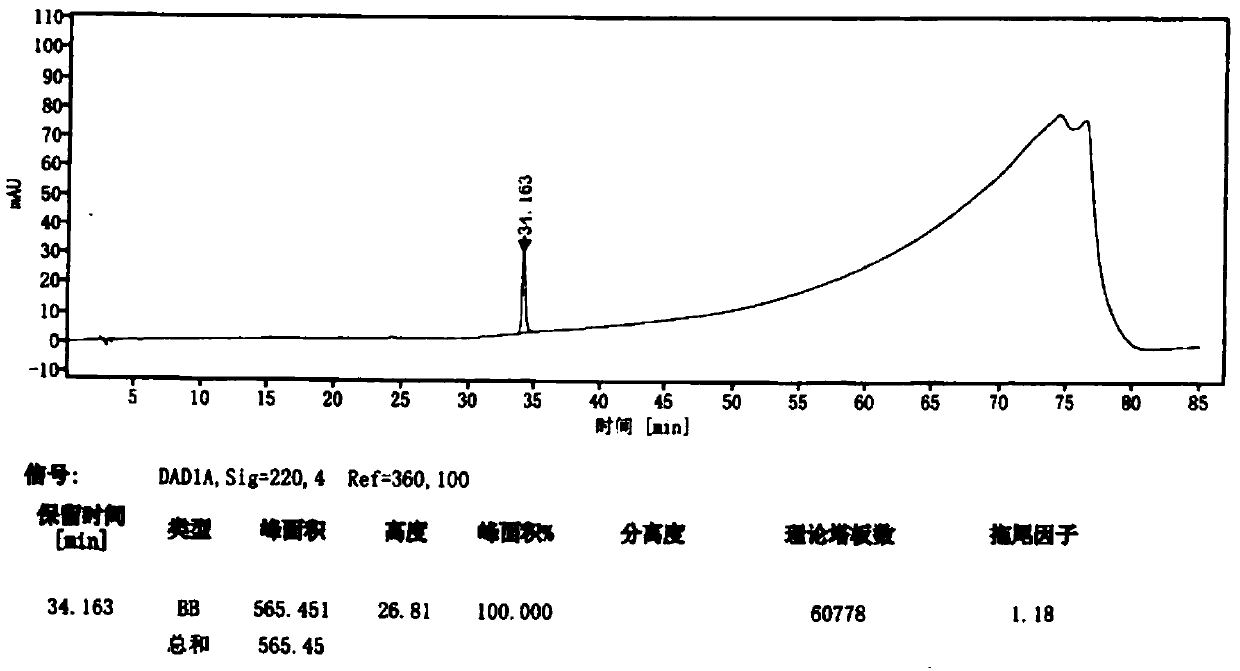
ActiveCN101710070ADetermination of contentEasy to separateComponent separationColor/spectral properties measurementsPhosphoric acidSolvent
The invention discloses a high performance liquid chromatography (HPLA) method for measuring content of hypocrellin A. The conditions of the liquid chromatography are as follows: octadecylsilane chemically bonded silica is used as a filler for a chromatographic column, and a mobile phase is a mixed solvent consisting of methanol and dilute phosphoric acid (or dilute formic acid, dilute acetic acid and dilute sulfuric acid), wherein the volume ratio of the methanol and the dilute phosphoric acid (or dilute formic acid, dilute acetic acid and dilute sulfuric acid) is 80:20-70:30, and the detection wavelength is 462 nm. The invention overcomes the defects of traditional analysis method and can be directly applied to the quantitative analysis of hypocrellin A in traditional Chinese medicine and preparation including the hypocrellin A. The method has the technical characteristics of good separation effect, precise measurement, sensitivity, strong specificity, fast measurement and the like. The absorption peak of the hypocrellin A in the medicine and preparation including the hypocrellin A can fully flow out the chromatographic column within 30 minutes, and the analysis work is finished.
Owner:YUNNAN PHYTOPHARML
Method for measuring contents of phosphorus, boron and arsenic impurities in silicon tetrafluoride gas
InactiveCN107957415AHigh purityEasy to produceAnalysis by thermal excitationTest samplePhysical chemistry
The invention discloses a method for measuring the contents of phosphorus, boron and arsenic impurities in silicon tetrafluoride gas. The method is characterized by comprising the following steps thata NaOH absorption solution is prepared; SiF4 which is purified after being absorbed by concentrated sulfuric acid, absorbed by concentrated sulfuric acid containing zeolite, absorbed by concentratedsulfuric acid and absorbed by concentrated sulfuric acid in sequence is introduced into an absorption bottle containing the NaOH absorption solution; the absorbed amount of the SiF4 gas is controlledto obtain a test sample, and the weight of the absorbed SiF4 gas is measured; the contents of phosphorus, boron and arsenic in the gas are analyzed by using the inductively coupled plasma atomic emission spectrometry.
Owner:GUIZHOU INST OF TECH
Chinese medicine combination with health protection function
ActiveCN101721472AAvoid infectionPromote absorptionComponent separationPill deliveryMedicineCurative effect
The invention relates to a Chinese medicine combination with a health protection function, comprising the following steps of firstly, decocting honeysuckles with water for 1-3 times to prepare a honeysuckle extractive; secondly, decocting scutellaria 1-3 times with water, combining decocted fluids and filtering, regulating pH of filtrate to 2-4, standing, leaching precipitations, washing with 40-70 percent ethanol and concentrating to prepare a scutellaria extractive; thirdly, respectively detecting contents of effective compositions in the honeysuckle extractive and the scutellaria extractive by using a liquid chromatography; and fourthly, preparing a yinhuang combination in a proportion, drying the yinhuang combination according to the needs of preparation forms, adding proper amount of auxiliary materials, and preparing into various preparation forms which can be clinically accepted through a conventional preparation method. The method solves the problem of difficult drying of a honeysuckle extractive thick paste, enhances the production efficiency and the utilization ratio of scutellaria so as to enable the concentration process to be easy to carry out, saves the energy and has remarkable curative effect by applying a yinhuang preparation including a yinhuang buccal tablet prepared by the method.
Owner:LUNAN HOPE PHARM CO LTD
Detection method for related substances in dexmedetomidine hydrochloride raw material or preparation
The invention relates to the technical field of drug detection and particularly relates to a detection method for related substances in a dexmedetomidine hydrochloride raw material or preparation. According to the method, dexmedetomidine hydrochloride and other inactive substances can be separated well and content of impurities can be accurately measured. According to the detection method providedby the invention, good propulsion effect for uniform establishment of quality control and standard of a product can be provided. According to the method, a high-performance liquid chromatography is employed; a detection wavelength is 215-220nm; a column temperature is 30-40 degrees centigrade; octadecyl silane bonded silica gel is taken as filler; acetonitrile-phosphate buffer is taken as mobilephase A; acetonitrile is taken as mobile phase B; trapping small columns are mounted between a gradient mixer and a sample injector; and gradient elution is carried out according to parameters set bythe method. According to the method, through adoption of the specific mobile phases and gradient elution parameters, the problem that the prior art is poor in separation degree and chromatographic column tolerance and high in cost is avoided.
Owner:石药银湖制药有限公司
Method for detecting content of optical isomers of bortezomib
ActiveCN103487526ADetermination of contentSolve the problem of content determinationComponent separationAmylaseBULK ACTIVE INGREDIENT
The invention discloses a method for detecting the content of optical isomers of bortezomib. The method is a high-performance liquid chromatography and comprises the steps as follows: a normal-phase chromatographic column with an amylase tris(3,5-dimethylphen-ylcarbamate) chiral stationary phase serving as a filler is adopted for coating the surface of silica gel, a mixed solvent of n-hexane, alcohol and an organic amine polarity modifier serves as a mobile phase, wherein the volume ratio of the n-hexane, the alcohol and the organic amine polarity modifier is (97-95):(3-5):(0-0.5), an ultraviolet detector is used for detection, the flow velocity is in a range of 1.0-1.4ml / min, and the temperature of the chromatographic column is in a range of 25-45 DEG C. According to the method, four optical isomers of the bortezomib can be well separated, so that the content of each optical isomer in the bortezomib can be measured accurately. The method is stable, reliable, high in sensitivity and applicable to quality control of bortezomib active ingredients and preparations of the bortezomib active ingredients in the research and production processes.
Owner:CHANGZHOU YABANG PHARMA
Method for simultaneously determining contents of three active components of ancient classical famous formula compound preparation Juanbi granules
PendingCN113325094ASimple methodAccurate methodComponent separationChlorogenic acidPhysical chemistry
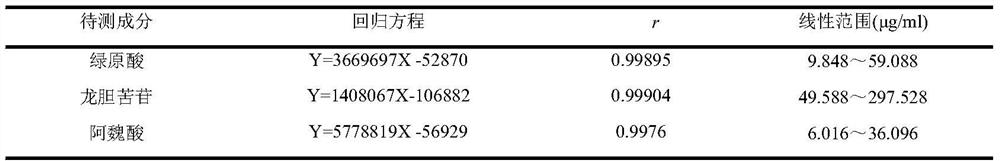
The invention discloses a method for simultaneously determining the contents of three active components of Juanbi granules as an ancient classical famous formula compound preparation. The chromatographic conditions adopted by the method are as follows, a chromatographic column is AgilentZORBAX SB-C18 (250mm*4.6 mm, 5 [mu] m), acetonitrile and 0.1% formic acid are used for gradient elution, the flow velocity is 1.0 ml.min<-1>, the detection wavelengths are as follows, chlorogenic acid and ferulic acid are 320nm, gentiopicroside is 277nm, the column temperature is 30 DEG C, and the sample size is 10 [mu] l. Under the chromatographic condition, the linear ranges of chlorogenic acid, gentiopicroside and ferulic acid are 9.848 to 59.088 [mu] g.ml<-1> (R is equal to 0.99895), 49.588 to 297.528 [mu] g.ml<-1> (R is equal to 0.99904) and 6.016 to 36.096 [mu] g.ml<-1> (R is equal to 0.9976) respectively, and the average sample recovery rates are 101.4% (RSD is equal to 2.4%), 97.2% (RSD is equal to 2.7%) and 100.0% (RSD is equal to 2.7%) (n is equal to 6) respectively. Chromatographic peaks of chlorogenic acid, gentiopicroside and ferulic acid are well separated, and concentration peak areas are in a good linear relationship. The method is simple, rapid, accurate and good in reproducibility, and a theoretical basis can be provided for comprehensively evaluating and controlling the quality of Juanbi granules as an ancient and classical famous formula compound preparation.
Owner:PHARMA FACTORY OF GUANGXI TRADITIONAL CHINESE MEDICAL UNIV
Method for measuring conductivity of inorganic binder sand
InactiveCN108956705AReach monitoringBasic assay contentMaterial resistanceDistilled waterRepeatability
The invention relates to a method for measuring the conductivity of inorganic binder sand. The method comprises the steps of adding distilled water into the inorganic binder sand, sequentially carrying out vibration and centrifugal separation, extracting supernate, and representing the amount of an inorganic binder in the inorganic binder sand by virtue of a method for detecting the conductivity of the supernate by virtue of a conductivity instrument. The method has the beneficial effects that the operation process is strict, simple and convenient; the stability is high; the numerical value ishigh, the repeatability is high, and meanwhile, the troubles in the preparation and use of a solution are reduced; and a chemical reagent is not used in the whole measurement process, and only wateris adopted, so that the method is relatively environment-friendly and efficient, and the cost is saved.
Owner:内蒙古仁创沙产业有限公司
Senegenin derivative, as well as preparation method and application thereof
InactiveCN102304164AAvoid end-absorption detection pitfallsDetermination of contentComponent separationSteroidsBenzoyl chlorideQuality control
The invention provides a senegenin derivative, as well as a preparation method and application thereof. In the senegenin derivative, the molecular formula is C44H52ClO8, and the name is 2,3-diphenyl formyloxy-senegenin. The preparation method comprises the following steps of: adding senegenin, dichloromethane, pyridine, benzoyl chloride and 4-dimethylamino pyridine into a reactor, stirring, and performing an ice water bath reaction; performing silicagel column chromatography and gel column chromatography to the prepared product; separating through semi-preparative high-efficiency liquid-phase chromatography; and collecting eluent of a corresponding chromatographic peak; concentrating in reduced pressure until the eluent is dry to obtain the senegenin derivative pure product with purity of over 98 percent. The pure product serving as a chemical reference substance can be used in content measurement of senegenin, and the ultraviolet maximum absorption wavelength of the senegenin derivative is 230nm and compared with 210nm of senegenin, so that the problems of specificity and stability in HPLC (High Performance Liquid Chromatography)-UV (ultraviolet) detection are solved. Therefore, the senegenin derivative, as well as the preparation method and application have practical values in quality control of the thinleaf milkwort rootbark medicinal material and thinleaf milkwort rootbark set prescription preparation.
Owner:SHANXI UNIV
Simple electrochemiluminescence detection method of glyphosate pesticide
InactiveCN107247079AQuantitative determination of contentDetermination of contentChemiluminescene/bioluminescenceMaterial electrochemical variablesCarbon nitrideElectrochemiluminescence
The invention belongs to the field of pesticide detection, and discloses a simple electrochemiluminescence detection method of glyphosate pesticide, and especially relates to preparation and applications of a carbon nitride-based glyphosate electrochemiluminescence sensor. According to a preparation method, graphene carbon nitride (g-C3N4) is used for modifying glassy carbon electrode loaded with Ag<+> on the surface; Ag<+> is released from the g-C3N4 modified electrode surface under the complexing action of glyphosate in a solution with Ag<+> on the surface of the electrode, recovery of electroluminescent signals is realized, and detection of glyphosate is realized via detecting the electroluminescent signals.
Owner:UNIV OF JINAN
Method for measuring content of elemental iodine complexed in povidone-iodine
InactiveCN105865883ADetermination of contentPreparing sample for investigationOrganic solventChemistry
The invention relates to a method for measuring the content of elemental iodine complexed in povidone-iodine, and particularly provides a method for measuring the content of elemental iodine complexed in povidone-iodine. According to the method, complexed iodine and non-complexed iodine in povidone-iodine are separated by using an organic solvent firstly, and then the content of complexed iodine in povidone-iodine is measured. According to the technical scheme of the invention, the content of complexed iodine in a povidone-iodine product can be really and effectively measured.
Owner:CHANGZHOU UNIV
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778955AReflect contentHighlight substantive featuresMicrobiological testing/measurementUltravioletWavelength
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Method for quantitatively determining content of cuprous oxide in corrosion products of copper
InactiveCN107315064ADetermination of contentAccurate measurementChemical analysis using titrationInorganic ChemicalSulfate
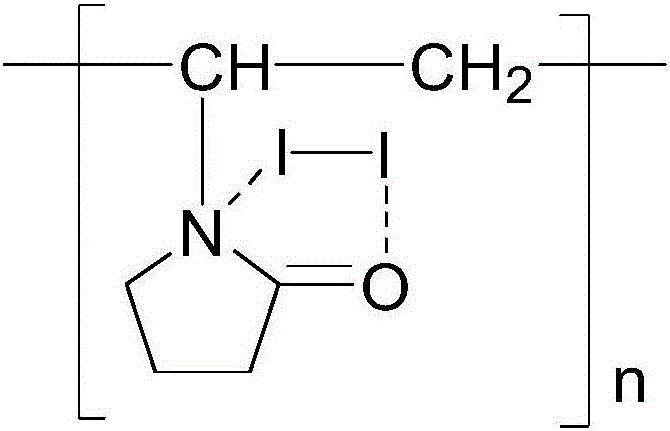
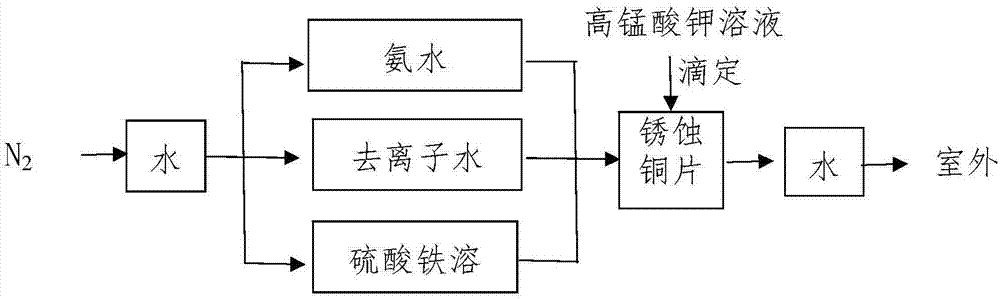
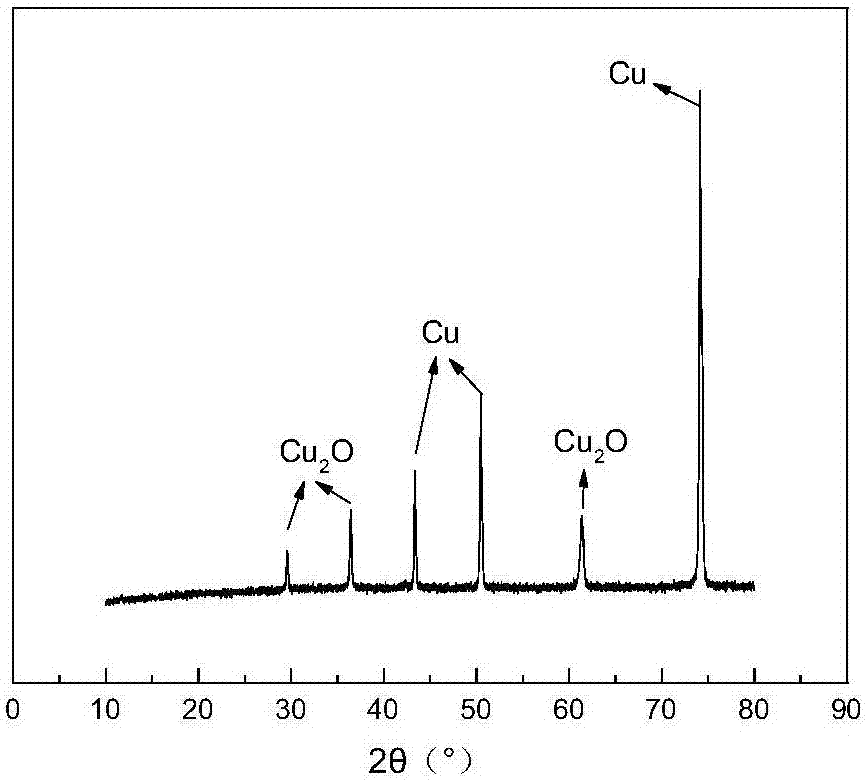
The invention specifically relates to a method for quantitatively determining the content of cuprous oxide in the corrosion products of copper, belonging to the technical field of inorganic chemical analysis. The method comprises the following steps: dissolving cuprous oxide on a to-be-detected sample in ammonia water under an anoxic condition, adding a ferric sulfate solution acidified by sulfuric acid after dissolving, and allowing ferric sulfate to oxidize cuprous ions into copper ion and to be reduced into ferrous sulfate; and titrating ferrous sulfate by using a standard potassium permanganate solution in an aerobic environment and calculating the content of cuprous oxide according to the content of ferrous sulfate. The method provided by the invention can quantitatively determine the content of cuprous oxide and has measurement error of + / - 3% or below. The method is applicable to research on the corrosion mechanism of copper.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method for detecting deep jet-flow sedimentary deposit based on natural gamma-ray spectrum logging
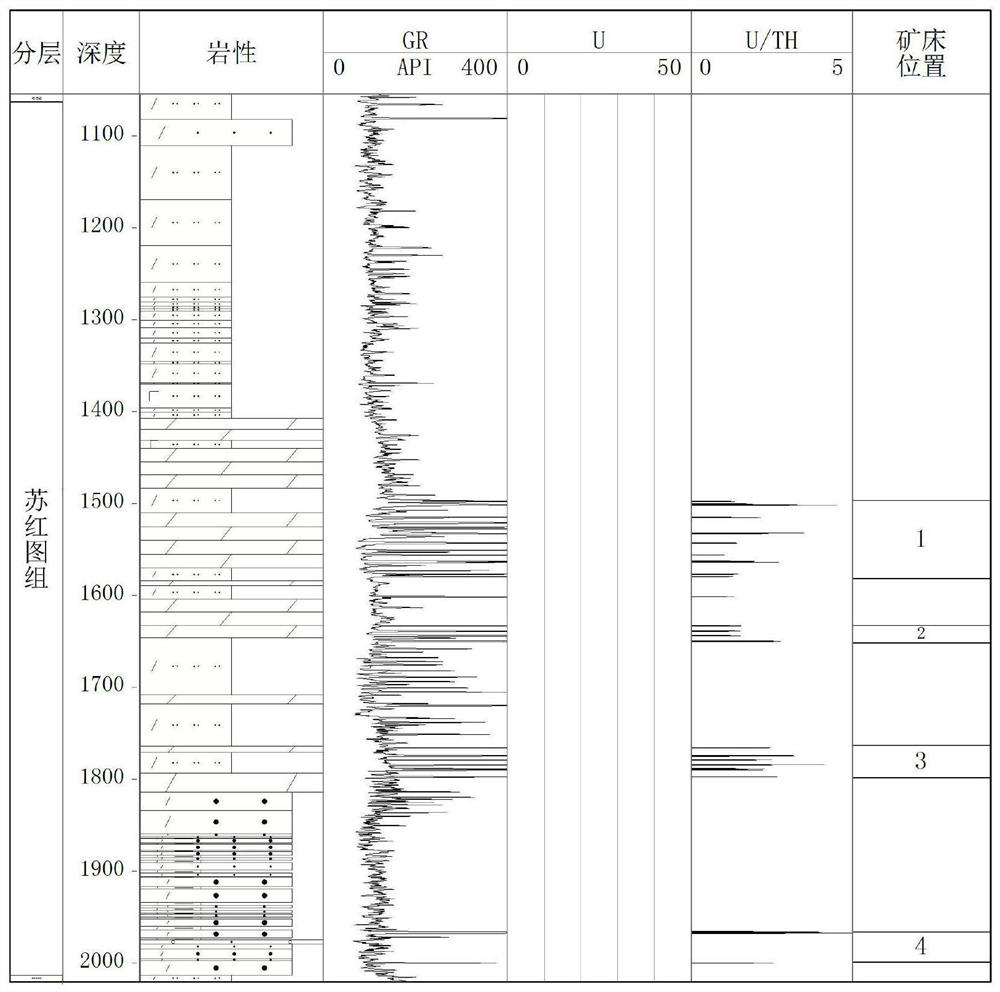
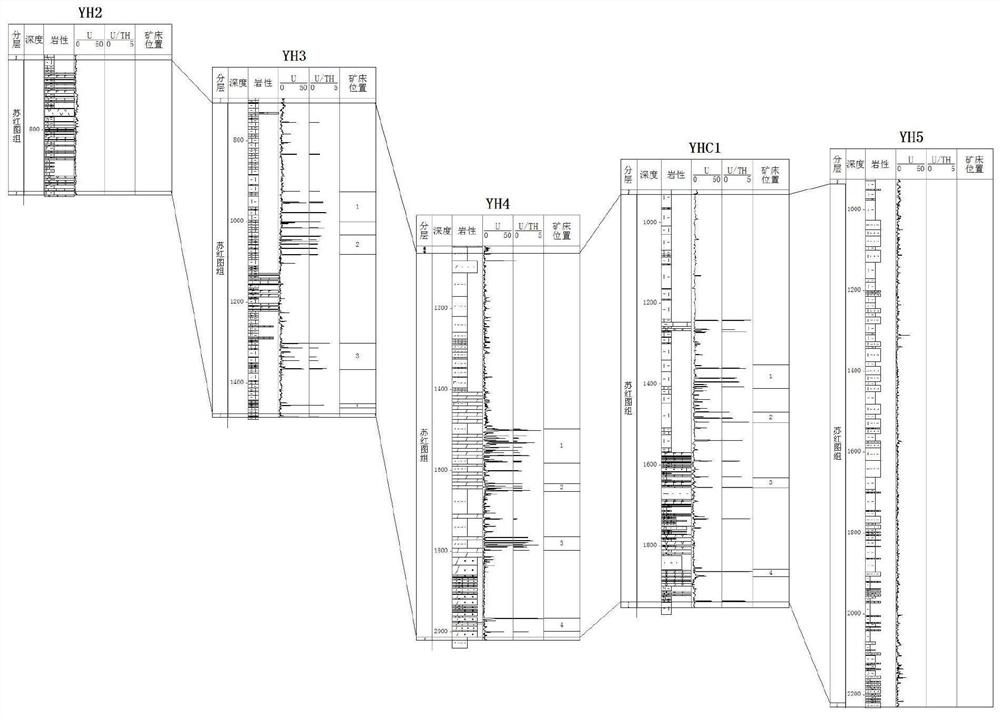
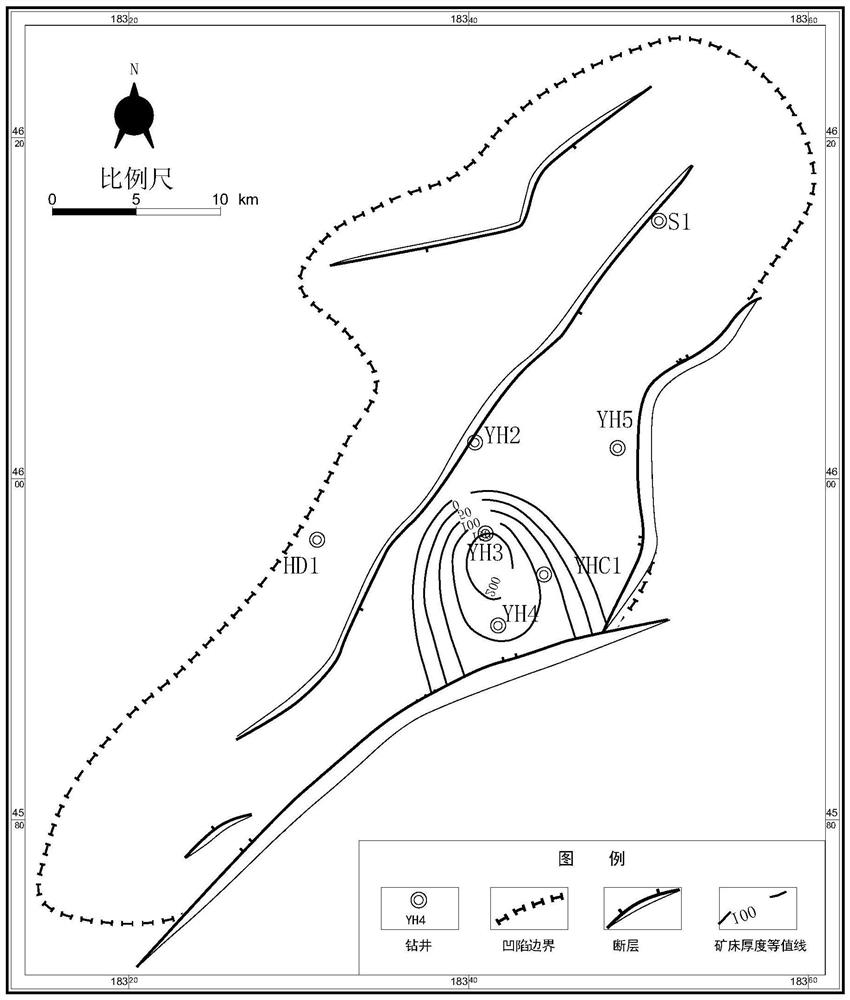
PendingCN113359203AWith enrichmentHas a high uranium to thorium ratioNuclear radiation detectionNuclear energy generationMetallogenyWell drilling
The invention provides a method for detecting a deep jet-flow sedimentary deposit based on natural gamma-ray spectrum logging. The method comprises the steps of 1, selecting a drilled well or a drilled hole; 2, calculating the contents of uranium and thorium elements in the stratum; 3, analyzing the contents of uranium and thorium elements, and screening out a high uranium layer section; 4, identifying the vertical distribution of the jet-flow sedimentary deposit; 5, determining the thickness, continuity and uniformity of the jet-flow sedimentary deposit; 6, determining the depth position, the distribution layer position and the thickness change of the jet-flow sedimentary deposit; and 7, determining the spatial form and the metallogenic law of the jet-flow sedimentary deposit, and predicting a favorable exploration area. According to the method, the uranium content and the thorium content in the stratum are obtained by utilizing the characteristics of uranium enrichment and high uranium-thorium ratio of the SEXDEX type ore deposit and combining natural gamma-ray spectrum logging analyzing, and a continuous longitudinal section of the uranium content and the uranium-thorium ratio is established; and the method provided by the invention is a method for rapidly, accurately and economically screening out favorable SEDEX ore deposit exploration targets in the deep stratum.
Owner:XI'AN PETROLEUM UNIVERSITY
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778941AHighlight substantive featuresSignificant progressMicrobiological testing/measurementPeroxidaseUltraviolet
The invention is about the measuring method of inorganic Phosphates and its diagnosis reagent box. Producing hydroperoxide by reacting pyruvate oxidase with pyruvate under the existence of Inorganic Phosphates , then causing enzyme-coupled reaction with peroxidase and oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length 400ú¡600nm absorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Calcium base desulfurater primary and secondary content and impurity element simultaneous determination method
ActiveCN101598673BThe area of acid rain is developing rapidlyAbundant resourcesPreparing sample for investigationAnalysis by thermal excitationSlurryDigestion
The invention relates to a calcium base desulfurater primary and secondary content and impurity elements simultaneous determination method, including the following steps: a calcium base desulfurater analysis sample is quantitatively weighed by a 180-200 mesh analysis sieve, a multi-step microwave digestion method is adopted for carrying out high temperature high pressure mixed acid microwave digestion on the quantitative calcium base desulfurater analysis sample, wherein the mixed acid is 2ml concentrated hydrochloric acid and 4-6ml concentrated nitric acid and the microwave digestion includes three steps, the sample after digestion is transferred for constant volume to prepare analysis solution, namely 2% dilute nitric acid is used for transferring the solution in the digestion inner pipe into a 25-50ml volumetric flask for constant volume to scale; and full spectrum direct-reading plasma emission spectrometer is used for accurately determining contents of multiple elements in the analysis solution. The invention not only can fast and accurately determine primary and secondary content and content of multiple elements in the calcium base desulfurater but also is applicable to determination of multiple elements in grout during desulfuration.
Owner:STATE GRID HEBEI ELECTRIC POWER RES INST +2
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778943AContent reflectionHighlight substantive featuresMicrobiological testing/measurementPeroxidaseUltraviolet
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing hypoxanthine by reacting nucleoside phosphorylase with carnine under the existence of Inorganic Phosphates in the sample of plasma, serum and so on, then producing urate and hydroperoxide by reactinghypoxanthine withxanthine oxidase, and then reacting bimolecular hydroperoxide with peroxidaseand oxidating the colorless reduced chromogen combination to quinoneimine chromogen or indamide chromogen dyer with color, testing the variation of dominant wave-length400ú¡600nmabsorbance during the reaction and finally measuring the content of Inorganic Phosphates . This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Hydrogen peroxide sensitive material and method for making same and application
InactiveCN101034063AHigh strengthDetermination of contentMaterial analysis by observing effect on chemical indicatorFluorescence/phosphorescenceReduction treatmentReducer
This invention belong to composite region, refer a hydrogen peroxide sensitive material and preparation method and its using in solution hydrogen peroxide detection. Hydrogen peroxide sensitive material possess TiO2 / SiO2 structure, Ti and Si in proper of 1 : 2-4. This hydrogen peroxide sensitive material could quickly and expediently, qualitative and quantitative detect drop hydrogen peroxide, and be able to reuse after using certain reducer to carry out reducing treatment, more economy.
Owner:SICHUAN UNIV
Aliskiren enantiomer content detecting method
InactiveCN104034825ADetermination of contentSolve the problem of content determinationComponent separationCelluloseCarbamate
The invention discloses an aliskiren enantiomer content detecting method which is a high performance liquid chromatography. The aliskiren enantiomer content detecting method comprises the steps that the surface of silica gel is coated with a normal-phase chromatographic column with a cellulose-three[3,5-xylyl carbamate] chiral stationary phase as padding, the mixed solvent of the normal hexane, the ethyl alcohol and the organic amine polarity modifier as the mobile phase, the volume proportion of the normal hexane, the ethyl alcohol and the organic amine polarity modifier is 90-94:6-10:0-0.5, detection is carried out through an ultraviolet detector, the flow velocity is 0.6-1.0ml / min, and the temperature of the chromatographic column ranges from 25 DEG C to 40 DEG C. According to the method, the aliskiren and the enantiomer corresponding to the aliskiren can be well separated, and therefore the content of the enantiomer of the aliskiren can be accurately measured, and the method can be used for carrying out quality control on the studying and producing process of the raw aliskiren medicine and the preparations of the raw aliskiren medicine.
Owner:常州市亚邦医药研究所有限公司 +3
Method of simultaneously determining content of three active components in eight-tradtional-medicine-herb Longzuan particles
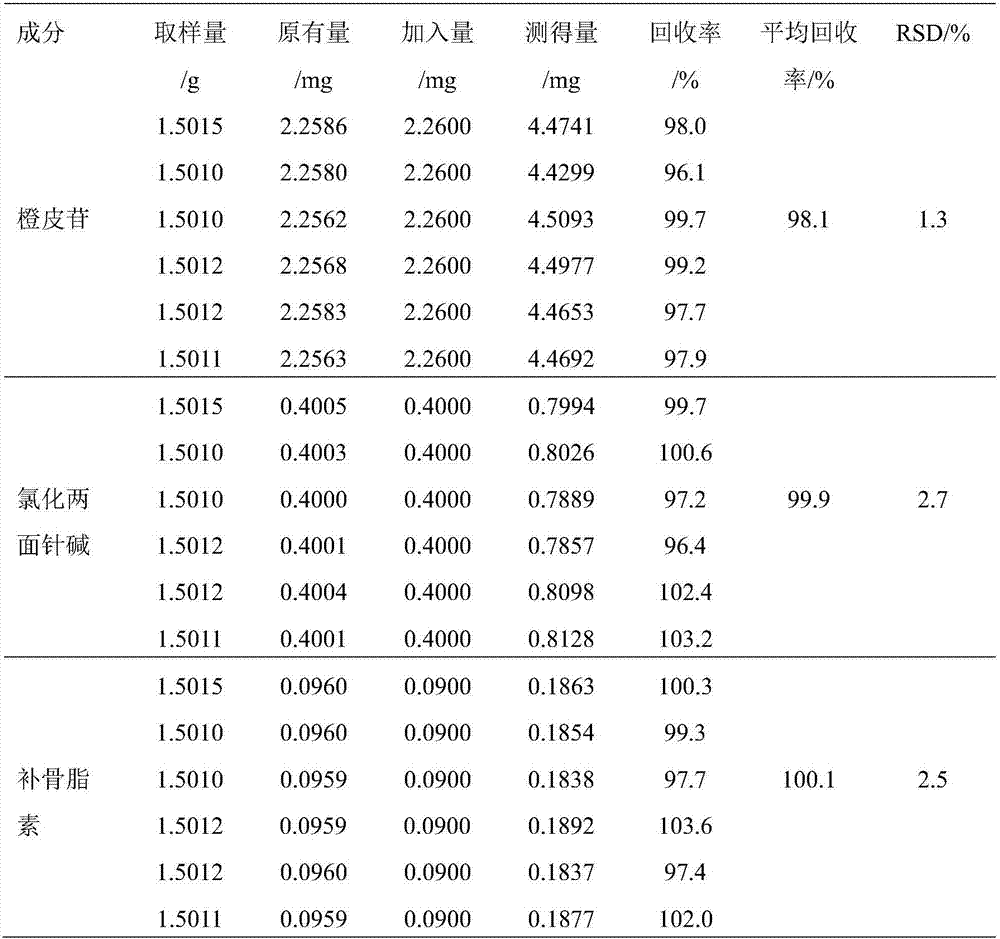
InactiveCN107543886ASimple methodAccurate methodComponent separationO-Phosphoric AcidColumn temperature
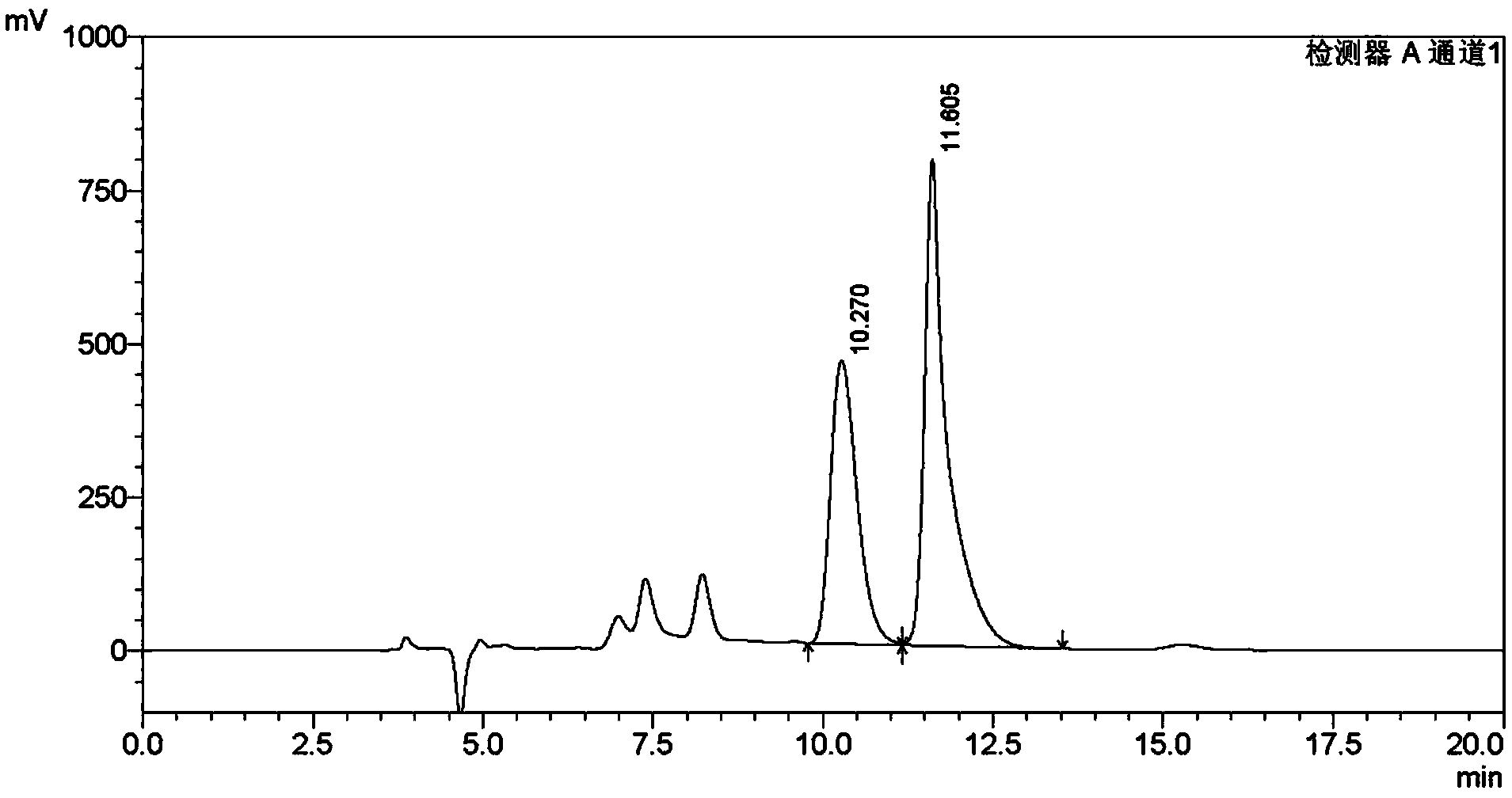
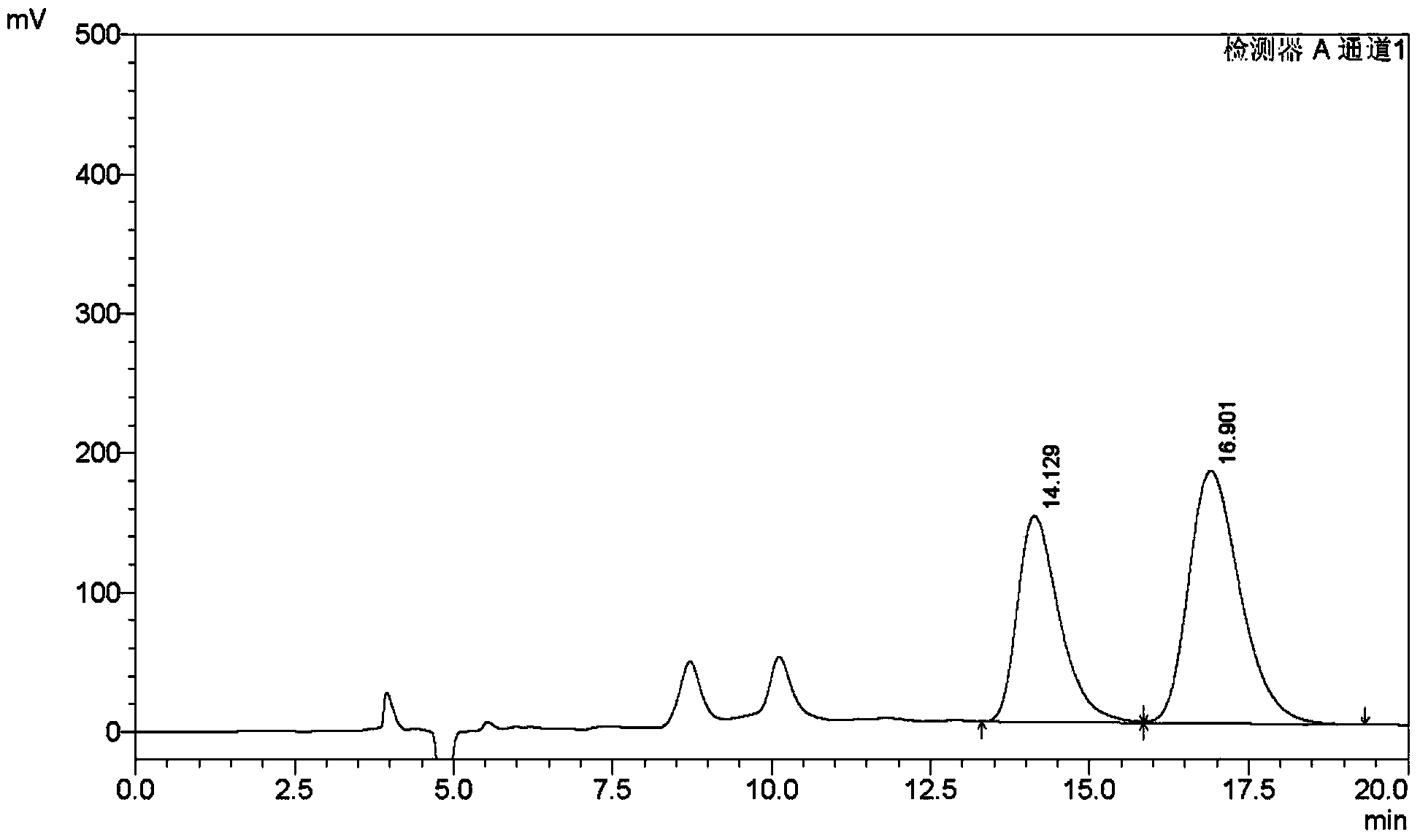
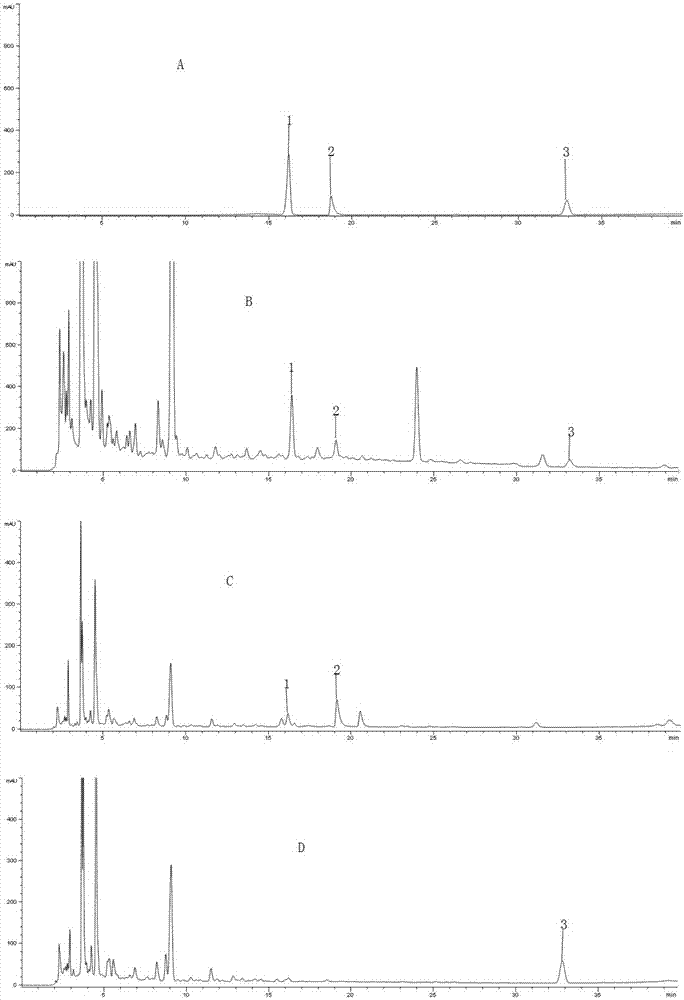
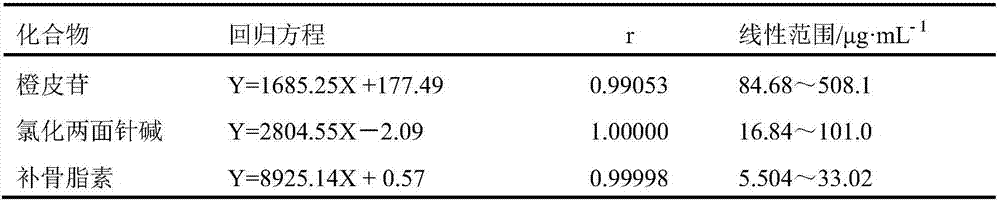
The invention discloses a method of simultaneously determining content of hesperidin, nitidine chloride and psoralen in eight-tradtional-medicine-herb Longzuan particles through HPLC. The chromatographic conditions adopted in the method are as follow: the specification of the chromatographic column Phenomenex Gemini C18 is (250 mm*4.6 mm, 5 micrometers), the mobile phase is acetonitrile, a 0.1% phosphoric acid water solution is used for gradient elution, the flow velocity is 1 ml / min, the detection wavelength is 286 nm and 245 nm (0-30 min: 286 nm, 30-40 min:245 nm), the column temperature is30 DEG C, and the sample size is 5 micrometers. Under the chromatographic conditions, chromatographic peaks are separated well, good linear relations are formed between hesperidin, nitidine chloride and psoralen and the respective peak areas in the concentration ranges of 84.68-508.1 microgram / milliliter,16.84-101.0 microgram / milliliter and 5.504-33.02 microgram / milliliter (r values are 0.99053, 1.00000 and 0.99998 respectively), the average recovery rates are 98.1%, 99.9% and 100.1% respectively, and the RSD values are 1.3%, 2.7% and 2.5% respectively (n=6). The method is simple, rapid, accurate, high in reproducibility and capable of providing a theoretical basis for comprehensively evaluating and controlling the eight-tradtional-medicine-herb Longzuan particles.
Owner:PHARMA FACTORY OF GUANGXI TRADITIONAL CHINESE MEDICAL UNIV
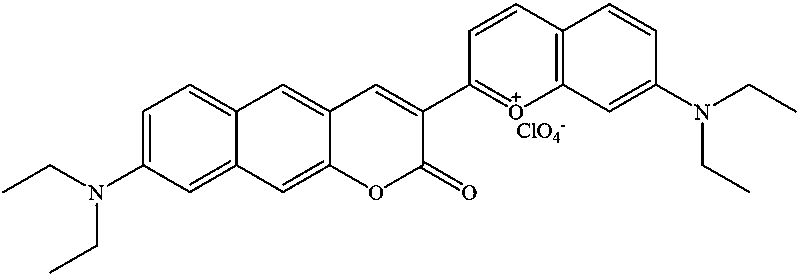
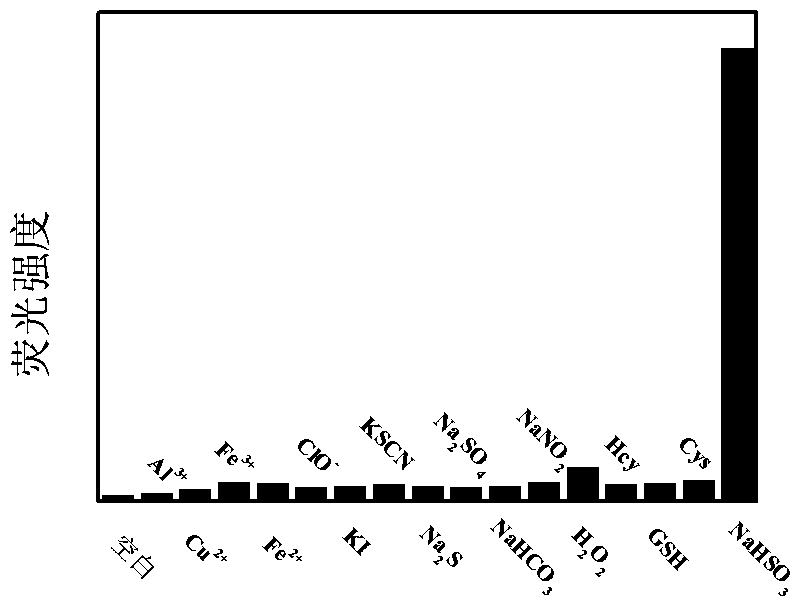
Deep infrared ratio type fluorescent probe capable of quickly responding to sulfur dioxide
ActiveCN111533725ARatiometric quantitative determination of contentDetermination of contentOrganic chemistryFluorescence/phosphorescenceFluorescenceSulfur dioxide
The invention discloses a fluorescent probe for detecting sulfur dioxide, and belongs to the technical field of analytical chemistry. The probe is prepared from prepared novel coumarin dye and 4-diethylamino salicylaldehyde in concentrated sulfuric acid at 90 DEG C. The preparation raw materials of the fluorescent probe are easy to obtain, the synthesis method is simple, and the fluorescent probehas the advantages of high selectivity, high sensitivity, low detection limit and the like for sulfur dioxide. The fluorescent probe can be applied to detection of sulfur dioxide in cells.
Owner:UNIV OF JINAN
Determination of inorganic phosphorus and its diagnostic reagent kit
InactiveCN1778954AReflect contentHighlight substantive featuresMicrobiological testing/measurementPhosphoenolpyruvate carboxylaseUltraviolet
The invention is about the measuring method of Inorganic Phosphates and its diagnosis reagent box. Producing carbon dioxide by reacting pyruvate oxidase with pyruvate under the activation ofInorganic Phosphates in the sample of plasma or serum and so on, and producing oxaloacetic acid by reacting carbon dioxide and phosphoenolpyruvate under the existence of phosphoenolpyruvate carboxylase, and then transferring oxidized coenzyme to reduced coenzyme by reacting oxaloacetic acid and malic acid dehydrogenase. Testing the descending range of dominant wave-length340nm absorbance and finally measuring the content of Inorganic Phosphates. This method has high specificity and would not be contaminated by material of internal and exogenous sources, and the result is precise and accurate. Diving the diagnosis reagent box into double-dose or three-dose can reduces the cross interaction of each element, keeps the stability of the reagent and deposits chronically. Using this method can realize the fast testing in common ultraviolet / visible light analyzer or semiautomatic / automatic analyzer and doesní»t require special or additional apparatus, so the cost is low. Thus, this method can be easily promoted and applied in the whole industry.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
A method for simultaneous determination of six active ingredients in Niuhuang Ninggong Tablets
InactiveCN104897787BSimple methodAccurate methodComponent separationPhosphoric acidColumn temperature
The invention discloses a method for simultaneous determination of six active components consisting of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride in a Niuhuang Ninggong tablet through HPLC. Chromatographic conditions employed in the invention are as follows: a chromatographic column is TC-C18 (4.6 mm * 250 mm, 5 [mu]m); detection wavelength is 280 nm; a mobile phase is methanol-0.05% phosphoric acid; gradient elution comprises three parts, i.e., elution with methanol with a concentration varying in a range of 10 to 80% in the time period from 0 min to 35 min, then elution with methanol with a concentration of 80% in the time period from 35 to 50 min, and finally elution with methanol with a concentration varying in a range of 80 to 10% in the time period from 50 to 60 min; flow velocity is 1.0 mL / min; column temperature is 25 DEG C; and sample size is 10 [mu]L. Under the above-mentioned chromatographic conditions, chromatographic peaks are perfectly separated, and concentrations and peak areas of chrysophanol, emodin, liquiritin, forsythin, baicalin and berberine hydrochloride show good linear relation. The method is simple, rapid and accurate, has good repeatability and can provide quality bases for comprehensive evaluation and control of the Niuhuang Ninggong tablet.
Owner:JILIN NORMAL UNIV
Method for detecting related substances in Tolterodine tartrate raw material or preparation
InactiveCN102368061ASignificant progressEfficient separationComponent separationAcetonitrileSilica gel
The invention relates to a method for detecting related substances in a Tolterodine tartrate raw material or preparation. The method employs high performance liquid chromatography (HPLC) for diction, adopts chromatographic column filled with cyano bonds and silica gel as an immobile phase, and takes a mixed solution of a phosphate buffer and acetonitrile. And the detection wavelength is 215nm. The method of the invention can effectively separate Tolterodine tartrate and other impurities well, detects impurities comprehensively within 215nm, and accurately determines the content of other impurities. Also, the method provided in the invention has the characteristics of simple operation, high sensitivity, accurate and reliable result as well as strong specificity.
Owner:LUNAN PHARMA GROUP CORPORATION
Mechanism for extracting dust in desulfurized flue gas, dust measuring device and method
InactiveCN111855321ADetermination of contentDispersed particle filtrationWithdrawing sample devicesThermodynamicsFlue gas
The invention discloses a mechanism for extracting dust in desulfurized flue gas, a dust measuring device and method. The dust extracting mechanism comprises a flue gas mixing box, a flue gas conveying and measuring assembly, a cooling assembly, a cleaning assembly and a drying assembly, wherein the flue gas conveying and measuring assembly is installed on the side face of the flue gas mixing boxand used for conveying flue gas to the flue gas mixing box and measuring the flow of the conveyed flue gas; the cooling assembly is installed on the side, away from the flue gas conveying and measuring assembly, of the flue gas mixing box and used for cooling flue gas in the flue gas mixing box to form dew; the cleaning assembly is communicated with the flue gas mixing box and used for cleaning dew dripped out of the flue gas mixing box to form sewage; and the drying assembly is used for filtering and drying the sewage to obtain dust. The mechanism has the beneficial effects that the minimum amount of dust brought out by the flue gas can be measured, whether the flue gas contains the dust or not can be verified, and the content of the dust in the flue gas can be measured.
Owner:ZAOZHUANG HUIFENG ENERGY TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com