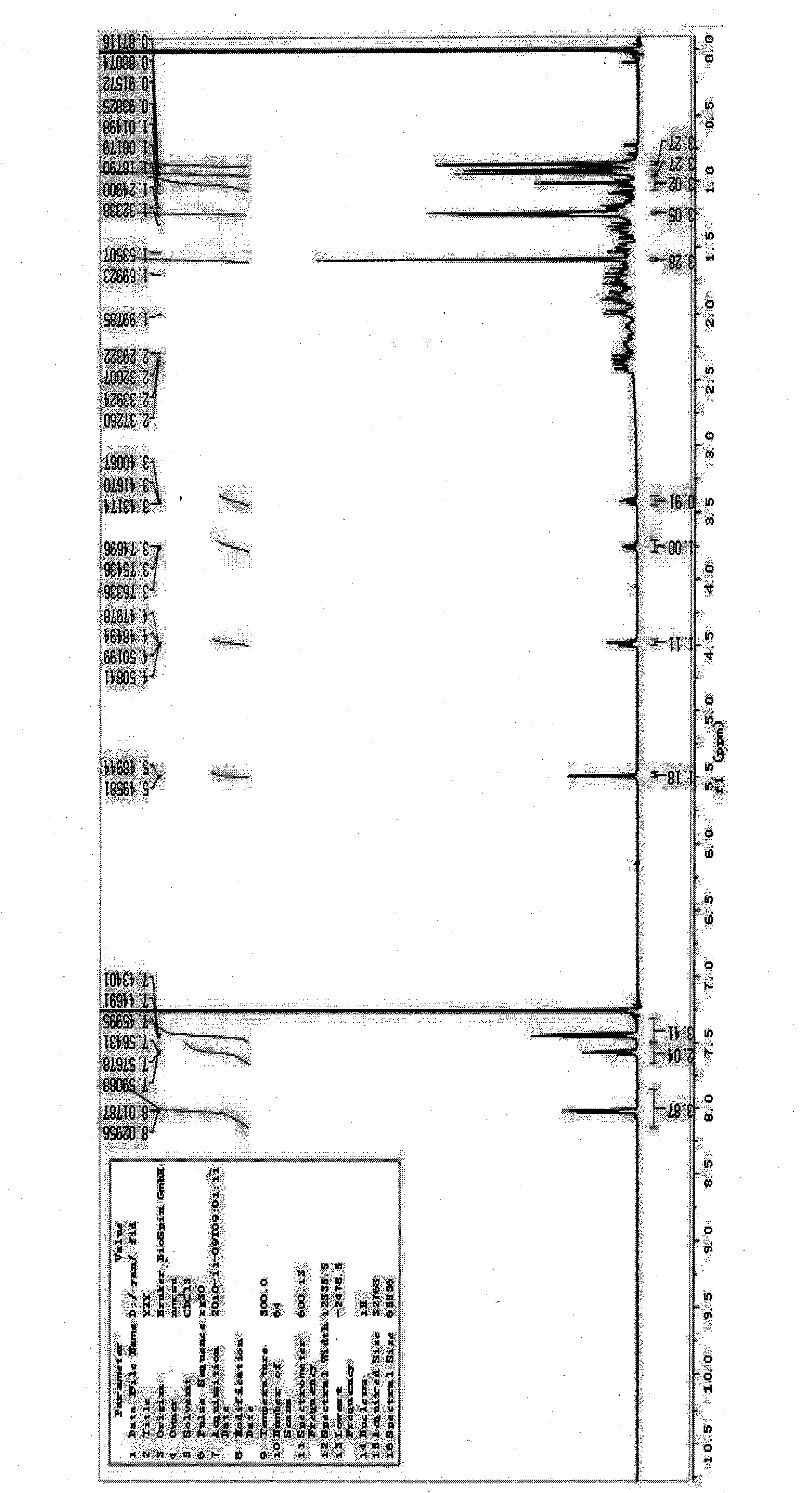
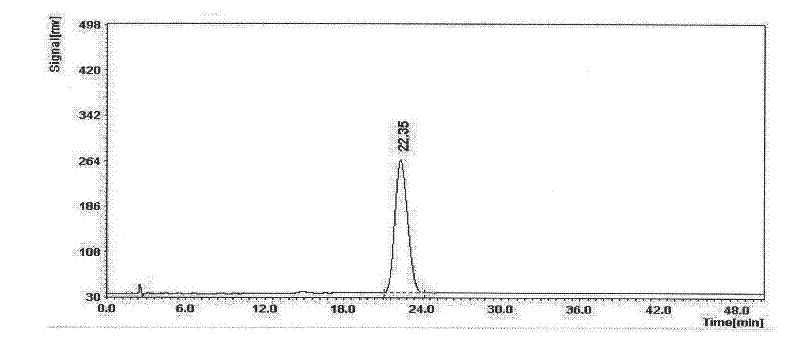
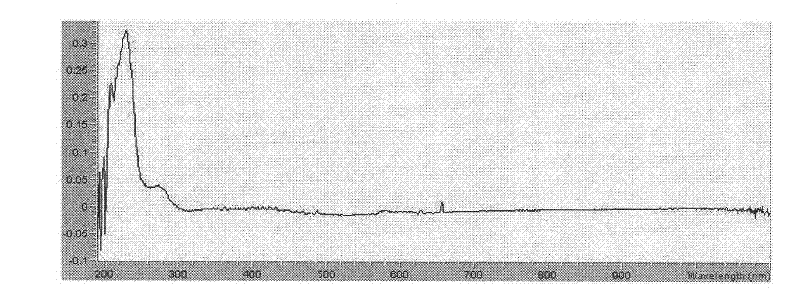
Senegenin derivative, as well as preparation method and application thereof
A technology of polysapogenin and derivatives, applied in steroids, measuring devices, instruments, etc., can solve the problems of large baseline drift, solvent interference, low sensitivity, etc., and achieve the effect of avoiding absorption detection defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Synthesis of Polysapogenin Derivatives
[0027] Weigh 0.1 g of polysapogenin, put it in a flask, add 15 mL of dichloromethane, 5 mL of pyridine, 4 mL of benzoyl chloride, 0.005 g of 4-dimethylaminopyridine, ice-water bath, and stir for 0.5 h to obtain crude polysaccharide derivative. .
[0028] (2) Separation by silica gel column chromatography
[0029] Take the silica gel, accurately weigh it, put it in an evaporating dish, slowly pour the crude polysapogenin derivative solution into the evaporating dish, stir with a glass rod, dry it, and accurately weigh it. Another appropriate amount of silica gel (200-300 mesh) was taken, swelled with petroleum ether-acetone (volume ratio 100:10), and packed into a column.
[0030] Load the sample, and sequentially use petroleum ether-acetone (volume ratios of 100:10, 100:15, 100:20, 100:25, 100:30, 100:35) to elute with a gradient of 3 column volumes each. The fractions of petroleum ether-acetone (volume ratio 100:35) were ...
Embodiment 2
[0037] (1) Synthesis of Polysapogenin Derivatives
[0038] Weigh 0.3 g of polysapogenin, put it in a flask, add 45 mL of dichloromethane, 10 mL of pyridine, 8 mL of benzoyl chloride, 0.005 g of 4-dimethylaminopyridine, stir in an ice-water bath, and stir with a magnetic stirrer for 1 h to obtain the derivative of polysapogenin. Crude goods.
[0039] (2) Separation by silica gel column chromatography
[0040] Weigh an appropriate amount of silica gel, accurately weigh it, place it in an evaporating dish, slowly pour the crude polysapogenin derivative solution into the evaporating dish, stir with a glass rod, dry it, and accurately weigh it. Take an appropriate amount of silica gel (200-300 mesh), swell with petroleum ether-acetone (volume ratio 100:10), and pack the column.
[0041] Load the sample, and sequentially use petroleum ether-acetone (volume ratios of 100:10, 100:15, 100:20, 100:25, 100:30, 100:35) to elute with a gradient of 3 column volumes each. The fractions of...
Embodiment 3
[0048] (1) Synthesis of Polysapogenin Derivatives
[0049] Weigh 0.5 g of polysapogenin, put it in a flask, add 75 mL of dichloromethane, 15 mL of pyridine, 12 mL of benzoyl chloride, 0.01 g of 4-dimethylaminopyridine, stir in an ice-water bath, and stir with a magnetic stirrer for 1 h to obtain the derivative of polysapogenin. Crude goods.
[0050] (2) Separation by silica gel column chromatography
[0051] Weigh an appropriate amount of silica gel, accurately weigh it, place it in an evaporating dish, slowly pour the crude polysapogenin derivative solution into the evaporating dish, stir with a glass rod, dry it, and accurately weigh it. Take an appropriate amount of silica gel (200-300 mesh), swell with petroleum ether-acetone (volume ratio 100:10), and pack the column.
[0052]Load the sample, and sequentially use petroleum ether-acetone (volume ratios of 100:10, 100:15, 100:20, 100:25, 100:30, 100:35) to elute with a gradient of 3 column volumes each. The fractions of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com