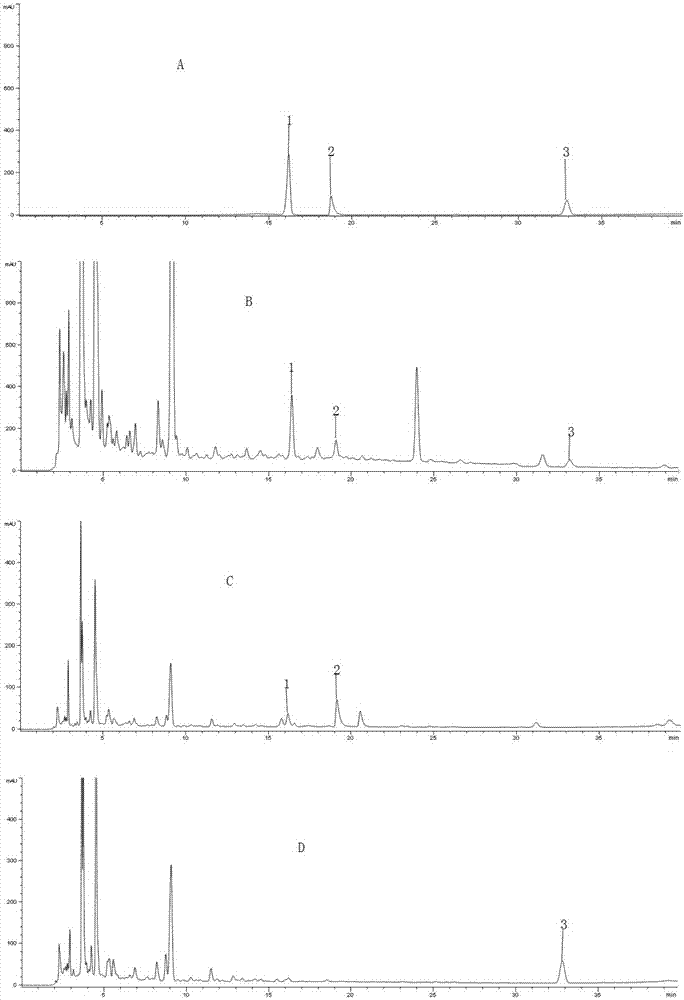
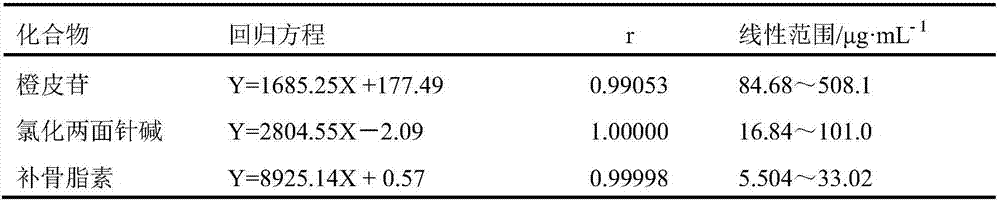
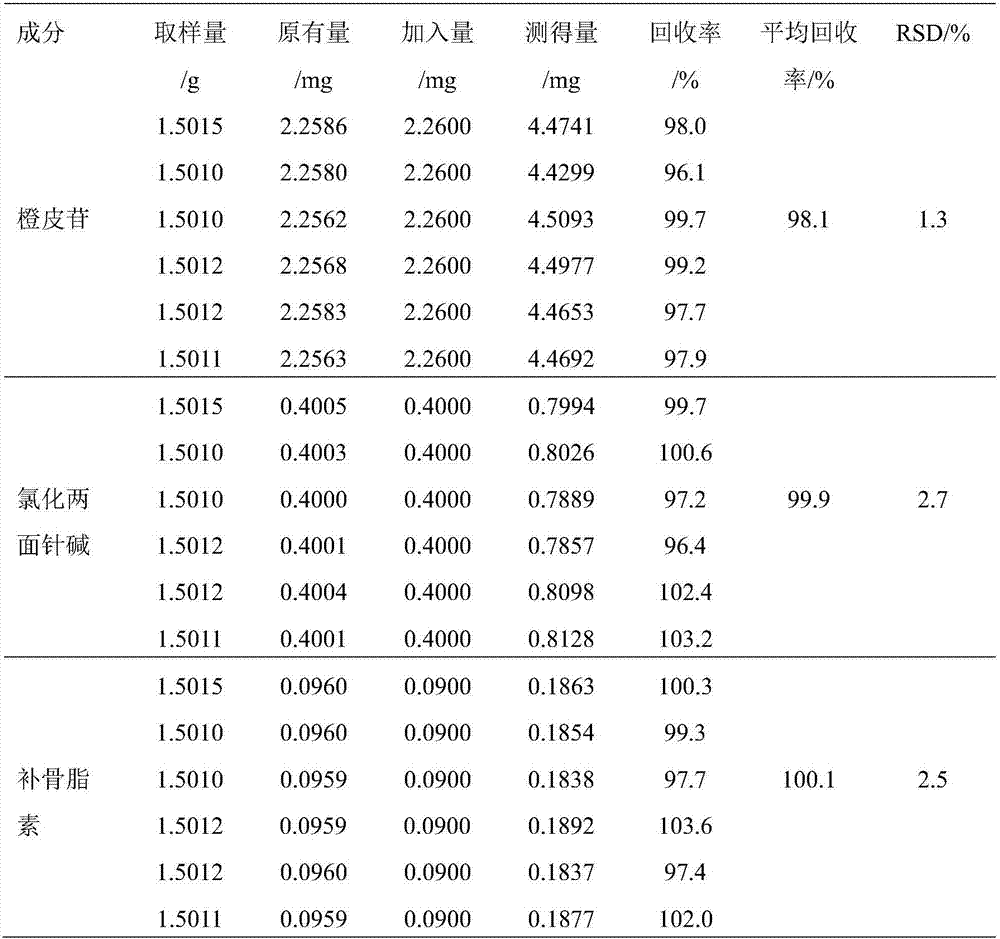
Method of simultaneously determining content of three active components in eight-tradtional-medicine-herb Longzuan particles
A particle and content technology, applied in the field of medicine, can solve the problems of difficulty in comprehensively and accurately controlling the quality of drugs, instability of other components, and few detection indicators, and achieves the effects of good reproducibility and stability, simple method and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments, so that those skilled in the art can better understand the present invention, but the present invention is not limited thereto.
[0022] 1 Materials and Instruments
[0023] 1.1 Materials The reference substance of acanthine chloride (MUST-16030407, content 98.3%) was purchased from the Chengdu Institute of Biology, Chinese Academy of Sciences, the reference substance of psoralen (for content determination, 110739-201617) and the reference substance of hesperidin ( For content determination, 110721-201617) were all purchased from the China Institute for Food and Drug Control; 10 batches of Baweilongzuan granules were provided by the Pharmaceutical Factory of Guangxi University of Traditional Chinese Medicine; all the original medicinal materials in the experiment were provided by the Pharmaceutical Factory of Guangxi University of Traditional Chin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com