Method for simultaneously determining contents of three active components of ancient classical famous formula compound preparation Juanbi granules
A technology for compound preparations and famous prescriptions, applied in the field of medicine, can solve rare problems, and achieve the effects of simple method, good reproducibility and stability, and good reproducibility and stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
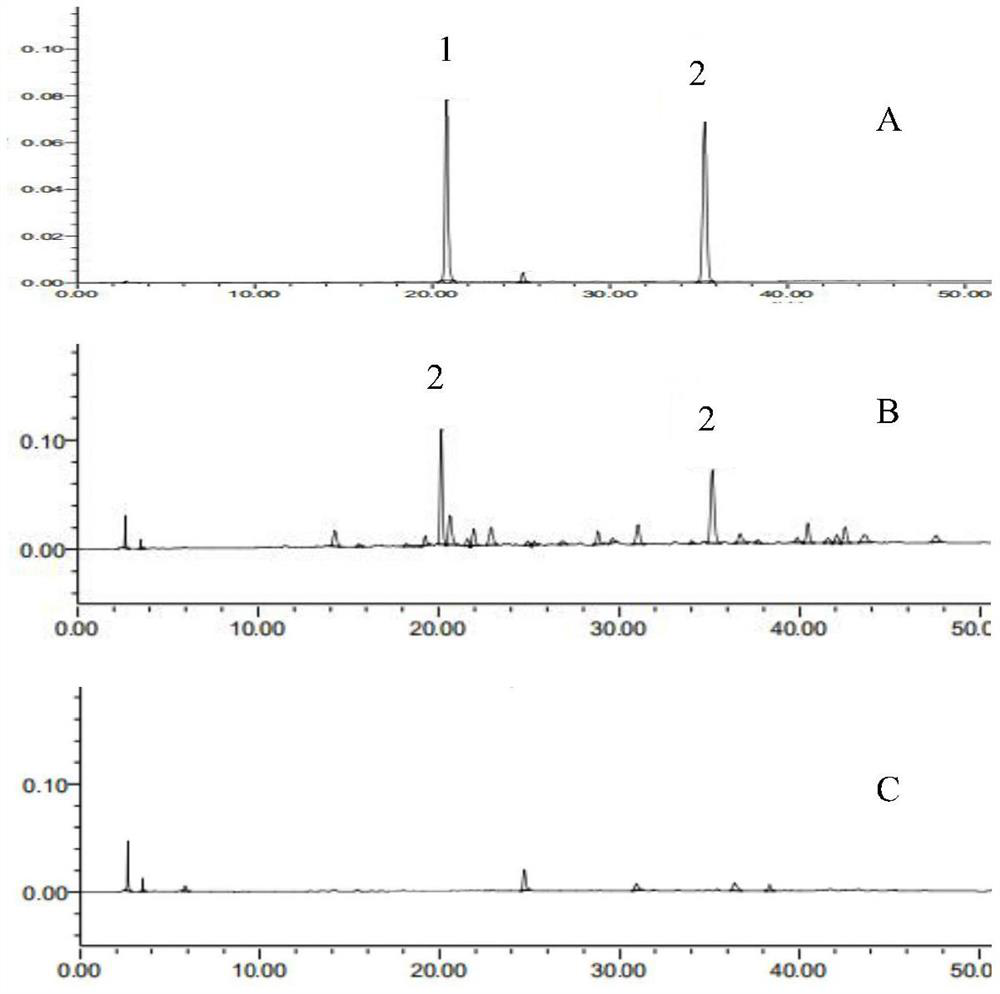
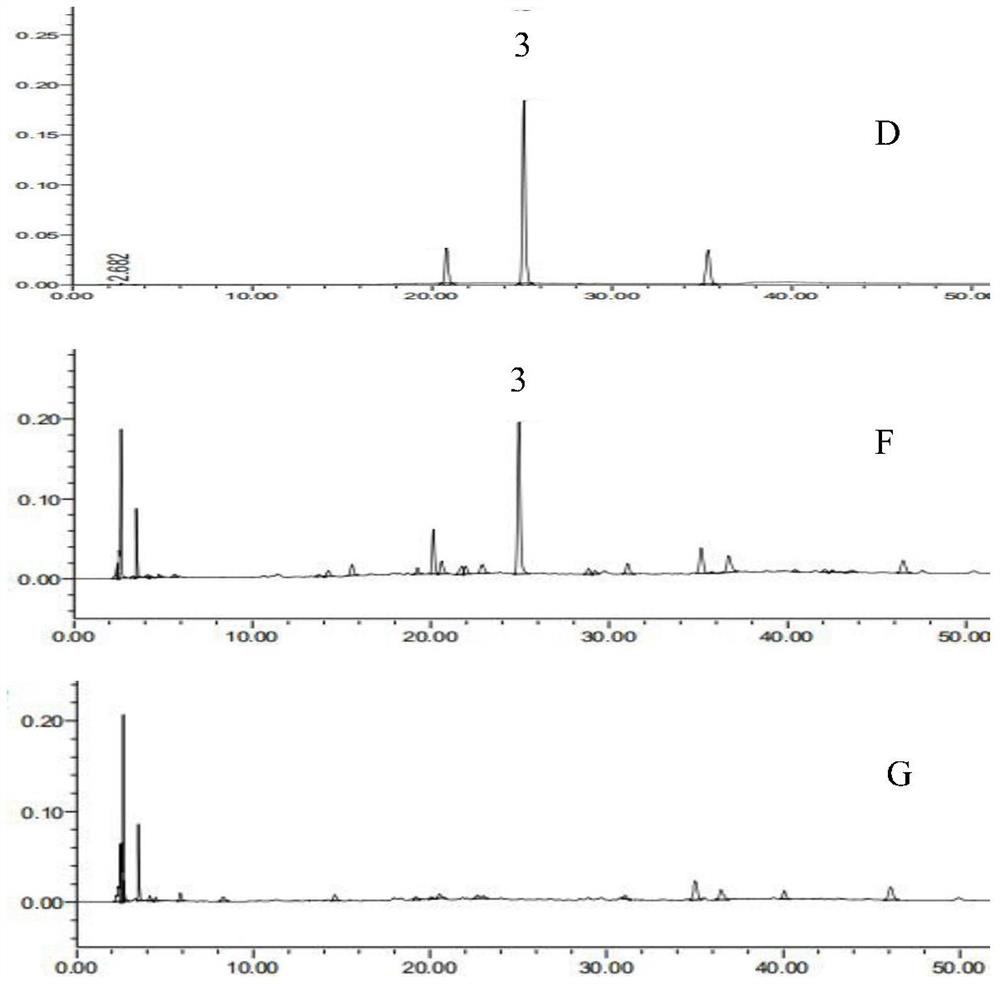
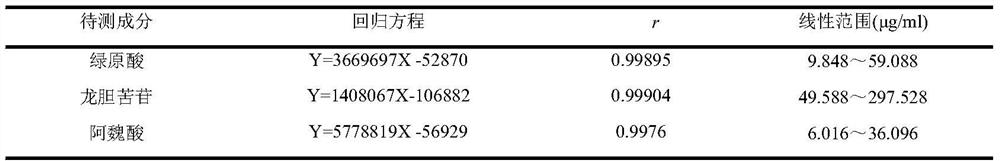
[0024] The present invention will be further described below in conjunction with the accompanying drawings and specific embodiments, so that those skilled in the art can better understand the present invention, but the present invention is not limited thereto.
[0025] 1 Instruments and reagents
[0026] 1.1 Instrument
[0027] American Waters-e2695 high-performance liquid chromatography (including quaternary high-pressure pump system, automatic sampler, UV detector and column thermostat, etc.); BT125D electronic balance (Sartorius Scientific Instrument Co., Ltd.); XS205 electronic balance (Mei Teller-Toledo); Ultrasonic cleaner (SG250HE, Shanghai Guante Ultrasonic Instrument Co., Ltd.); Electric constant temperature water bath (HH-4, Changzhou Aohua Instrument Co., Ltd.); Prepare to wait.
[0028] 1.2 Reagent
[0029] Reference substance: chlorogenic acid (Chlorogenic acid) (B20782, content 98%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd., Gentiopicroside (f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com