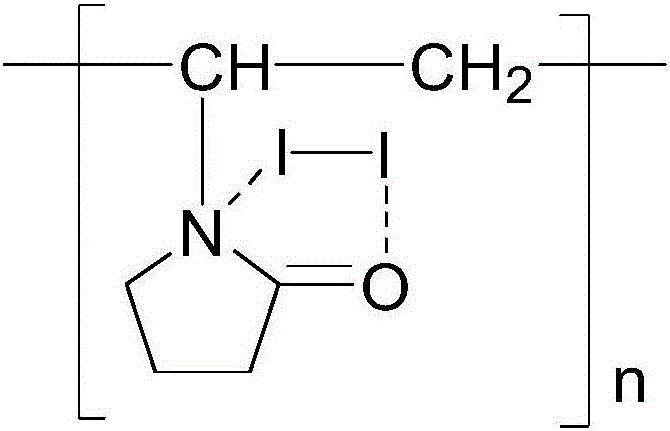
Method for measuring content of elemental iodine complexed in povidone-iodine
A technology of povidone iodine and its determination method, which is applied in the field of determination of the content of elemental iodine complexed in povidone iodine, can solve the problem of the inability to determine the content of complexed iodine, the inability to determine the slow-release property, iodine and polyethylene There is no way to judge the quality of pyrrolidone complexation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 4.00g of pharmaceutical grade polyvinylpyrrolidone K30 and 1.00g of iodine into a three-necked flask equipped with a stirrer and a reflux condenser, add 10.0mL of ethanol to dissolve, heat the oil bath to 70°C, stir and reflux for 2 hours to prepare PVP-I. The prepared PVP-I sample was transferred to a 100mL volumetric flask and made to volume with distilled water. Then use a pipette to pipette 5.00 mL of the above constant-volume PVP-I solution, put it in a one-necked flask, add 25.00 mL of cyclohexane solution, and stir it electrically for 1 h. After the stirring was stopped, the mixture in the one-necked flask was transferred to a separatory funnel. After standing to separate layers, the cyclohexane layer and the PVP-I solution layer were placed in conical flasks respectively. Use the calibrated 0.1mol / L Na 2 S 2 o 3 The standard solution was titrated for two samples. The analysis result is that the upper cyclohexane solution contains 12.44 mg of iodine, an...
Embodiment 2
[0023] Add 4.00g of pharmaceutical grade polyvinylpyrrolidone K30 and 1.00g of iodine into a three-necked flask equipped with a stirrer and reflux condenser, add 10.0mL of ethanol to dissolve, heat the oil bath to 70°C, stir and reflux for 4 hours to prepare PVP-I. The prepared PVP-I sample was transferred to a 100mL volumetric flask and made to volume with distilled water. Then use a pipette to pipette 5.00 mL of the above constant-volume PVP-I solution, put it in a one-necked flask, add 25.00 mL of cyclohexane solution, and stir it electrically for 1 h. After the stirring was stopped, the mixture in the one-necked flask was transferred to a separatory funnel. After standing to separate layers, the cyclohexane layer and the PVP-I solution layer were placed in conical flasks respectively. Use the calibrated 0.1mol / L Na 2 S 2 o 3 The standard solution was titrated for two samples. The result of the analysis is that the upper cyclohexane solution contains 9.90 mg of iodine...
Embodiment 3
[0025] Add 6.00g of pharmaceutical grade polyvinylpyrrolidone K30 and 1.00g of iodine into a three-necked flask equipped with a stirrer and reflux condenser, add 10.0mL of ethanol to dissolve, heat the oil bath to 60°C, stir and reflux for 6 hours to prepare PVP-I. The prepared PVP-I sample was transferred to a 100mL volumetric flask and made to volume with distilled water. Then use a pipette to pipette 5.00 mL of the above constant-volume PVP-I solution, put it in a one-necked flask, add 25.00 mL of cyclohexane solution, and stir it electrically for 1 h. After the stirring was stopped, the mixture in the one-necked flask was transferred to a separatory funnel. After standing to separate layers, the cyclohexane layer and the PVP-I solution layer were placed in conical flasks respectively. Use the calibrated 0.1mol / L Na 2 S 2 o 3 The standard solution was titrated for two samples. The result of analysis is that the upper cyclohexane solution contains 7.61 mg of iodine, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com