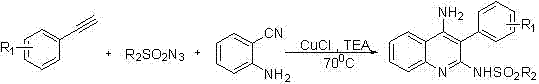
Method used for preparing 4-aminoquinoline derivative
A technology of aminoquinoline and derivatives, which is applied in organic chemistry and other fields, can solve problems such as troublesome post-processing, cumbersome process, and complicated process, and achieve good industrial application prospects, improve reaction efficiency, and simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
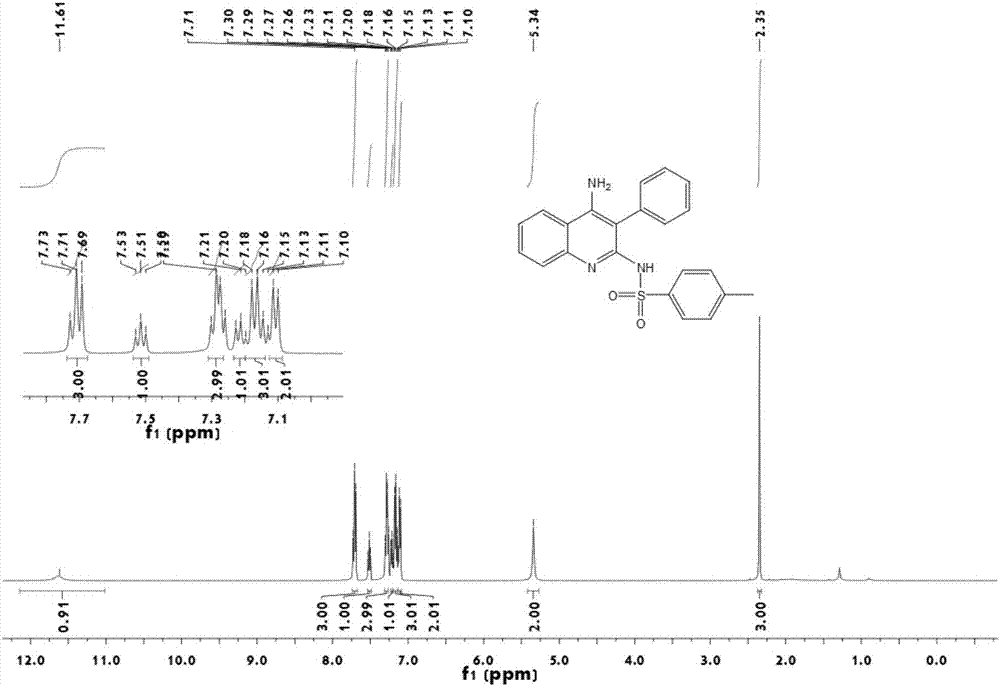
[0020] Add 2mg of CuCl catalyst, 23mg of o-aminobenzonitrile, 22μL of phenylacetylene and 20μL of p-toluenesulfonyl azide into a 10ml reaction tube, then add 1ml of 1,4-dioxane, and replace 40mg of triethylamine with nitrogen Add dropwise to the reaction tube. The reaction mixture was heated at 70°C for 4 h, the mixture was filtered, washed three times with petroleum ether / ethyl acetate (volume ratio 5 / 1, 10 mL), the filtrate was combined and concentrated, and separated by column chromatography, the eluent was petroleum ether / ethyl acetate Ethyl acetate (volume ratio 2 / 1) was 600ml to obtain the final product N-(4-amino-3-phenyl-quinolin-2-yl)-4-methylbenzenesulfonamide with a yield of 85%.
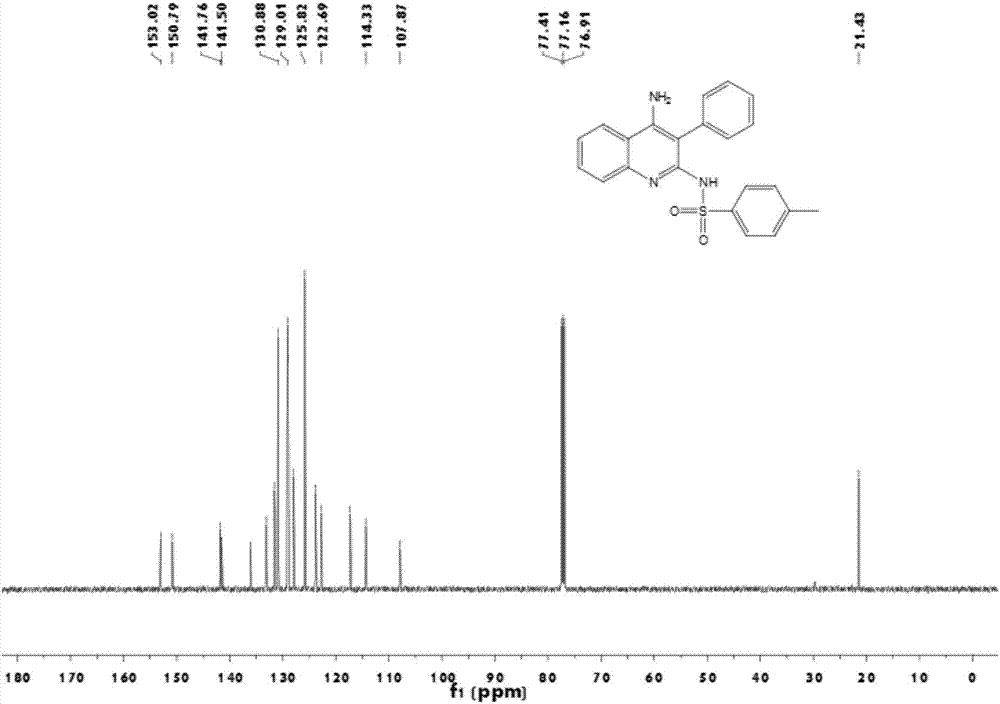
[0021] 1 H NMR (500 MHz, CDCl 3 ) δ = 11.61 (s, 1H), 7.71 (t, J=10.0 Hz, 3H),7.51 (t, J=7.5 Hz, 1H), 7.29 (t, J=7.5 Hz, 3H), 7.21 (t, J=7.5 Hz, 1H), 7.20– 7.14 (m, 3H), 7.11 (d, J=7.5 Hz, 2H), 5.34 (s, 2H), 2.35 (s, 3H); 13 C NMR (126 MHz, CDCl 3 ) δ = 153.0, 150.8, 141.8, 141.5, 136...
Embodiment 2
[0024] Add 2 mg of CuCl catalyst, 23 mg of o-aminobenzonitrile, 39 μL of 4-methoxyphenylacetylene and 20 μL of p-toluenesulfonyl azide into a 10 ml reaction tube, then add 1 ml of 1,4-dioxane, and replace with nitrogen Add 40 mg of triethylamine dropwise to the reaction tube. The reaction mixture was heated at 70°C for 4 h, the mixture was filtered, washed three times with petroleum ether / ethyl acetate (volume ratio 5 / 1, 10 mL), the filtrate was combined and concentrated, and separated by column chromatography, the eluent was petroleum ether / ethyl acetate Ethyl acetate (volume ratio 2 / 1) 600ml to obtain the final product N-[4-amino-3-(4-methoxyphenyl)-quinolin-2-yl]-4-methylbenzenesulfonamide, The yield was 42%.
[0025] 1 H NMR (500 MHz, DMSO) δ = 11.42 (s, 1H), 8.22 (d, J=10.0 Hz, 1H),7.73 (d, J=10.0 Hz, 1H), 7.68 – 7.57 (m, 3H), 7.35 (t, J=7.5 Hz, 1H), 7.26(d, J=10.0 Hz, 2H), 7.03 (q, J=10.0 Hz, 4H), 6.69 (s, 2H), 3.81 (s, 3H), 2.31(s, 3H); 13 C NMR (126 MHz, DMSO) Δ =...
Embodiment 3
[0027] Add 2 mg of CuCl catalyst, 23 mg of o-aminobenzonitrile, 25 μL of 3-methylphenylacetylene and 20 μL of p-toluenesulfonyl azide into a 10 ml reaction tube, then add 1 ml of 1,4-dioxane, and replace with nitrogen 40 mg of triethylamine was added dropwise into the reaction tube. The reaction mixture was heated at 70°C for 4h, the mixture was filtered, washed three times with petroleum ether / ethyl acetate (volume ratio 5 / 1, 10mL), the filtrate was combined and concentrated, and separated by column chromatography, the eluent was petroleum ether / ethyl acetate Ethyl acetate (volume ratio 2 / 1) was 600ml to obtain the final product N-(4-amino-3-m-tolyl-quinolin-2-yl)-4-methylbenzenesulfonamide with a yield of 68%.
[0028] 1 H NMR (500 MHz, CDCl 3) δ = 11.72 (s, 1H), 7.73 (d, J=10.0 Hz, 2H),7.69 (d, J=5.0 Hz, 1H), 7.56 (t, J=7.5 Hz, 1H), 7.32 (d, J=10.0 Hz, 1H), 7.26– 7.17 (m, 4H), 7.09 (d, J=5.0 Hz, 1H), 6.95 (s, 2H), 5.24 (s, 2H), 2.37 (s,3H), 2.29 (s, 3H); 13 C NMR (126 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com