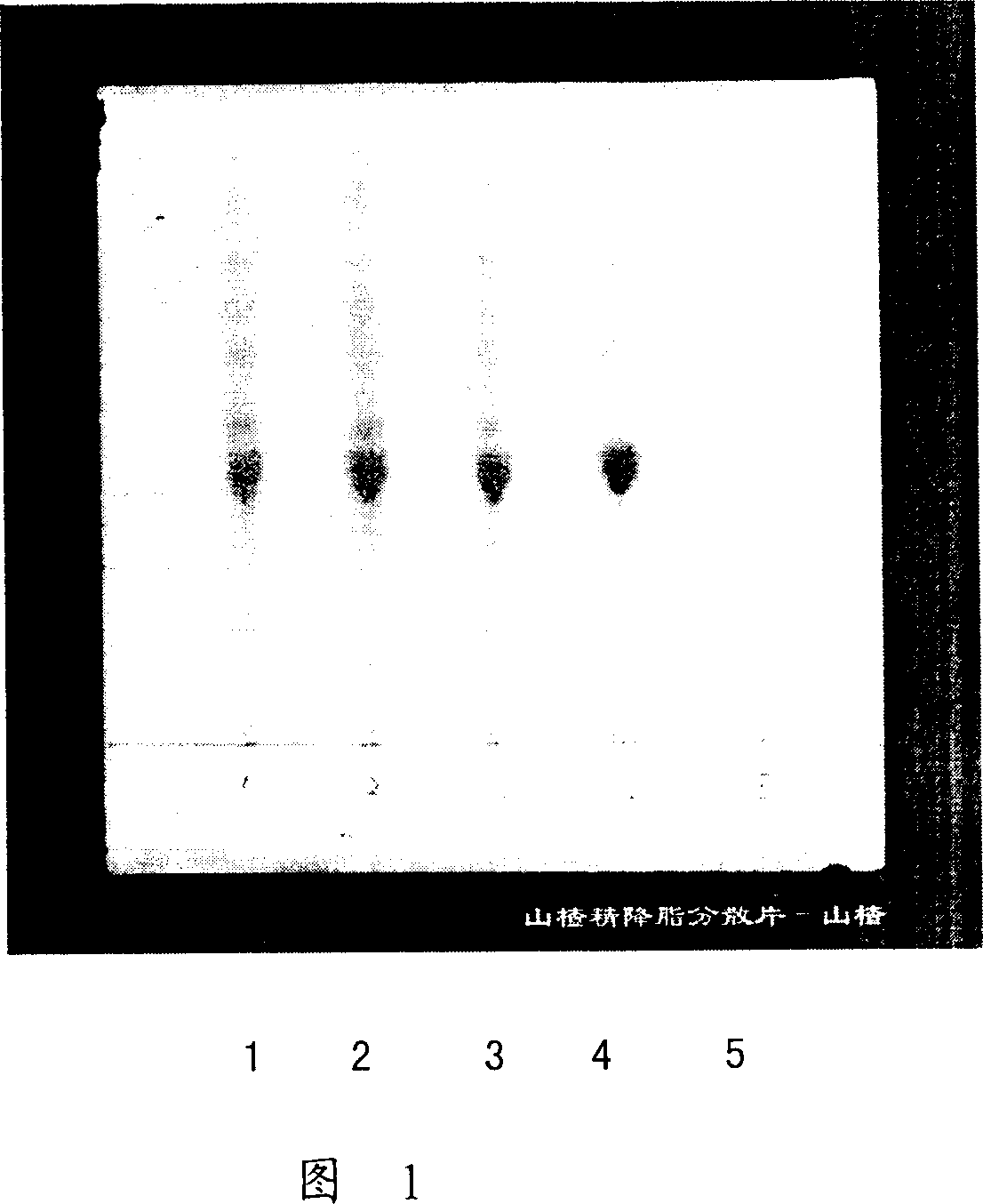
Method for identifying hawthorn of hawthorn extract lipid-lowering dispersion tablet
A technology of dispersible tablets and hawthorn, which is applied in the directions of material separation, analysis of materials, and analysis by chemical reaction of materials, can solve problems such as poor specificity, and achieve the effects of simple operation, good separation effect and clear spots.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1. Preparation of the test solution:
[0016] Take 0.8g of three batches of hawthorn essence lipid-lowering dispersible tablets with batch numbers 040713, 040714, and 040715, grind them finely, add 30ml of ether, extract under reflux for 1 hour, let cool, filter, evaporate the filtrate to dryness, add 2ml of methanol to the residue to dissolve, As the test solution.
[0017] 2. Preparation of the reference drug solution
[0018] Take 0.5 g of hawthorn reference medicinal material, and make a reference medicinal material solution according to the preparation method of the test solution.
[0019] 3. Preparation of Hawthorn-deficient Negative Reference Substance Solution
[0020] In addition, all auxiliary materials were weighed according to the prescription amount, and 1000 dispersible tablets were made according to the preparation process of Hawthorn Essence Jiangzhi Dispersible Tablets, which was the negative reference substance lacking hawthorn, and 0.6g of this prod...
Embodiment 2
[0025] 1. Preparation of the test solution:
[0026] Take 0.5g of three batches of hawthorn essence lipid-lowering dispersible tablets with batch numbers 040713, 040714, and 040715, grind them finely, add 20ml of petroleum ether, reflux and extract for 30 minutes, let cool, filter, evaporate the filtrate to dryness, add 2ml of methanol to dissolve the residue , as the test solution.
[0027] 2. Preparation of reference solution
[0028] Take 0.5 g of hawthorn reference medicinal material, and make a reference medicinal material solution according to the preparation method of the test solution.
[0029] Take ursolic acid, add methanol to make a solution containing 1mg per 1ml.
[0030] 3. Preparation of Hawthorn-deficient Negative Reference Substance Solution
[0031] In addition, all auxiliary materials were weighed according to the prescription amount, and 1000 dispersible tablets were made according to the preparation process of Hawthorn Essence Jiangzhi Dispersible Table...
Embodiment 3
[0036] 1. Preparation of the test solution:
[0037] Take 2.0g of three batches of hawthorn essence lipid-lowering dispersible tablets with batch numbers 040713, 040714, and 040715, grind them finely, add 100ml of n-hexane, reflux and extract for 1 hour, let cool, filter, evaporate the filtrate to dryness, add 2ml of methanol to dissolve the residue , as the test solution.
[0038] 2. Preparation of the reference drug solution
[0039] Take 0.5 g of hawthorn reference medicinal material, and make a reference medicinal material solution according to the preparation method of the test solution.
[0040] Take ursolic acid, add methanol to make a solution containing 1mg per 1ml.
[0041] Take oleanolic acid, add methanol to form a solution containing 1mg per 1ml.
[0042] 3. Preparation of Hawthorn-deficient Negative Reference Substance Solution
[0043] In addition, all auxiliary materials were weighed according to the prescription amount, and 1000 dispersible tablets were ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com