Tinidazole tablet consistency evaluation method
A technology of tinidazole tablets and evaluation methods, which is applied in the direction of instruments, measuring devices, scientific instruments, etc., can solve the problems of consuming a lot of time and cost, affecting the progress of research and development, etc., and achieve saving time and cost, improving production efficiency, and good dissolution rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] An embodiment of a method for evaluating the consistency of tinidazole tablets provided by the present invention includes in vitro dissolution curves and BE tests, and also includes a pre-evaluation process. The pre-evaluation process includes crystal form, impurity comparison, and dissolution curve determination. Dissolution profiles and BE tests were performed before.
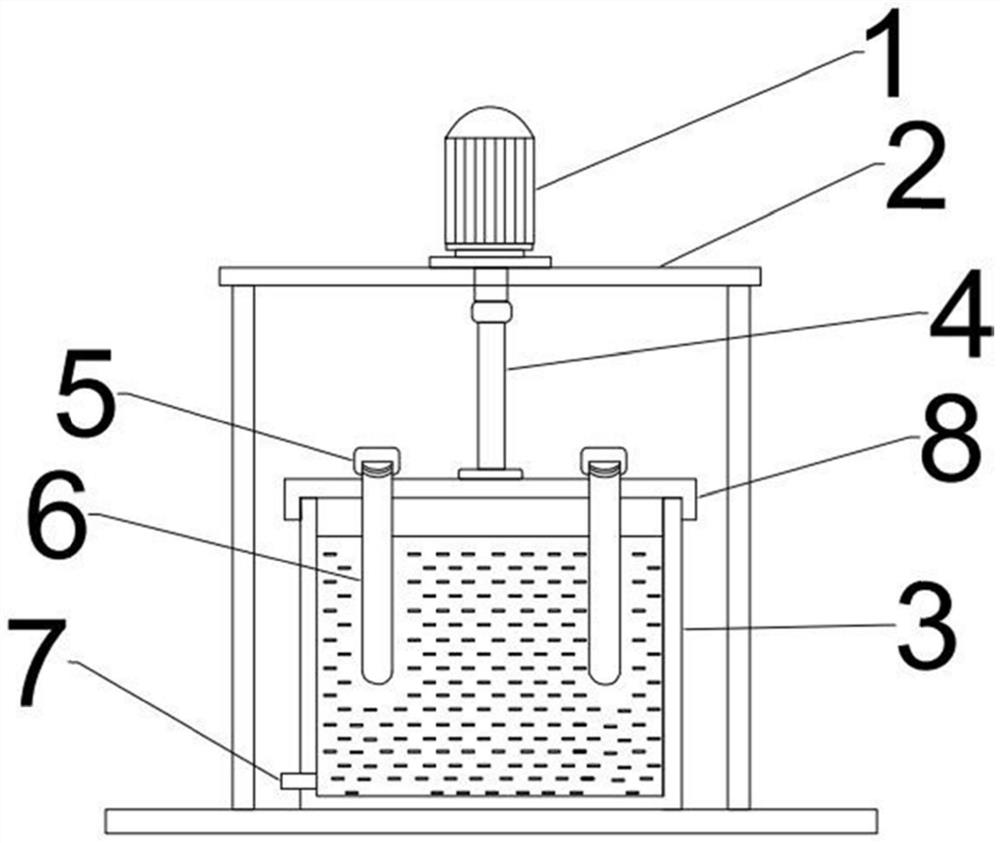
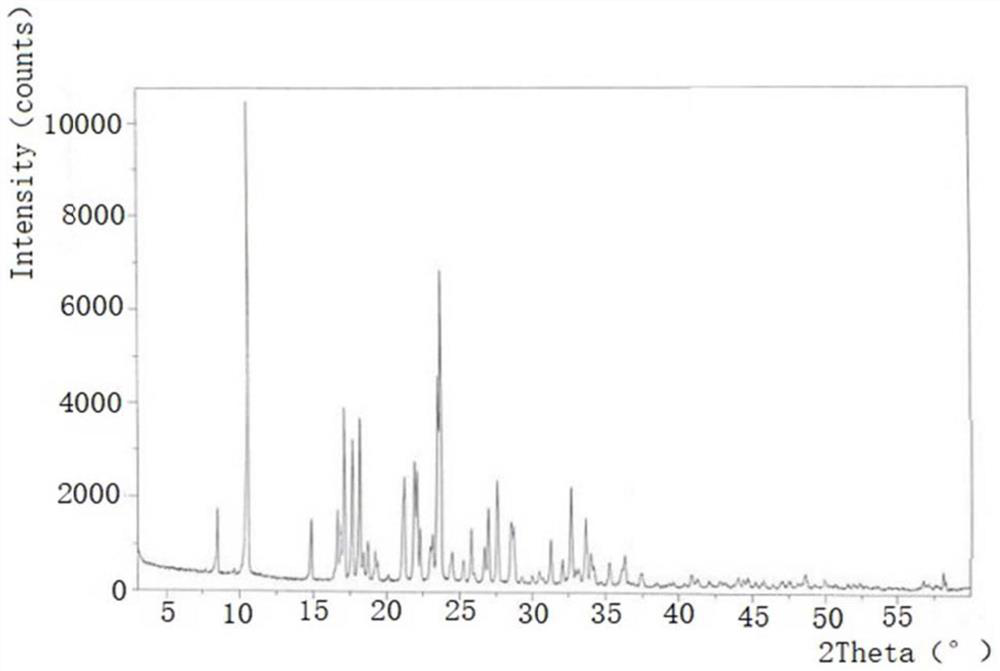
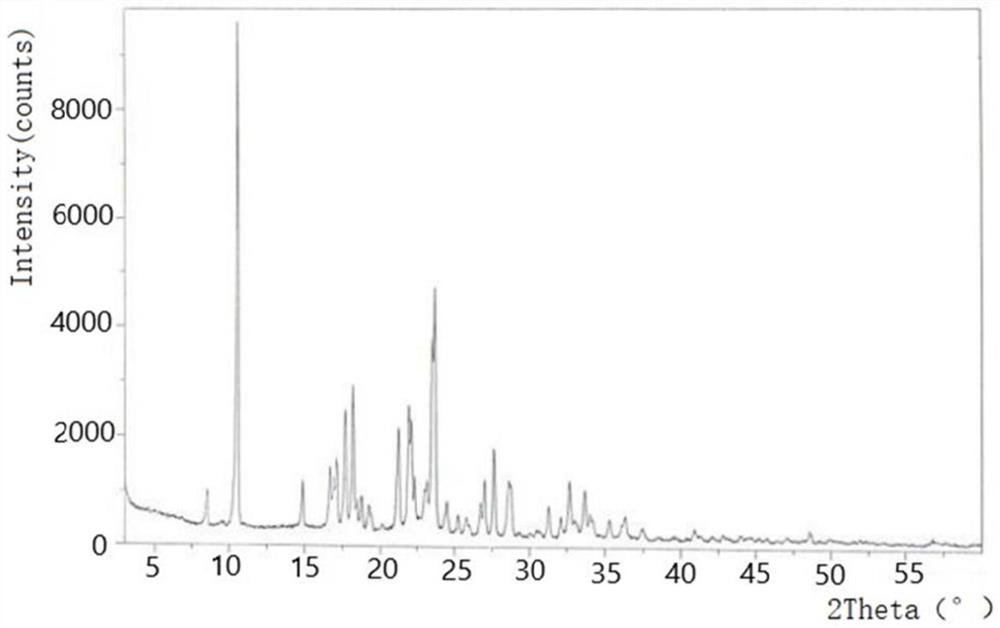
[0035] Comparison of crystal forms using powder diffraction method to compare tinidazole tablets and reference preparations Compared with the crystal forms, one piece of tinidazole tablet (content is 0.5g) and one piece of reference preparation (the content is 0.5g) After being ground into powder respectively, they are pressed into flat tablets in a tablet machine, and the flat tablets are respectively put into an X-ray diffractometer to measure the spectrograms of the two drugs to determine the crystal form.
[0036] It should be noted that powder X-ray diffraction is used to identify the crystal f...
Embodiment 2
[0054] In an embodiment provided by the present invention, the tinidazole tablet to be tested includes a tablet core and a coating, and the tablet core includes 180 parts by weight of tinidazole, 90 parts by weight of a diluent, 12 parts by weight of a binder, and 15 parts by weight of a disintegrant. part, 3 parts by weight of lubricant.
[0055] Prepared by the following process:
[0056] (1) Preparation of adhesive solution: take pregelatinized starch and disperse it evenly in an appropriate amount of purified water, place it in a water bath heating at 90-95°C and stir continuously to make it gelatinized, and the product is obtained;
[0057] (2) Premixing: add tinidazole, diluent, and disintegrant croscarmellose sodium into the mixer for mixing every 3 to 4 minutes, in which the diluent is microcrystalline cellulose and pregelatin a mixture of starches;
[0058] (3) Granulation: Turn on the boiling drying granulator, spray the binder solution prepared in step (1) for gra...
Embodiment 3
[0064] As a further improvement to Example 1, in the comparison of crystal forms, it was observed that tinidazole tablets and If the characteristic peaks of the spectrum are inconsistent, it means that the two currently measured drug crystal forms are inconsistent. You can choose to replace the raw materials of tinidazole tablets from different manufacturers and repeat the production of tinidazole tablets, and then conduct powder diffraction samples to determine whether the crystal forms are consistent. The drug properties of nidazole tablets are changed, and the raw materials and production process are excluded for tinidazole tablets and reference preparations The impact of consistency evaluation; if the crystal form is inconsistent, it means that the efficacy of tinidazole tablets itself is different from that of the reference preparation The efficacy of tinidazole tablets is inconsistent, and the production process of tinidazole tablets needs to be improved again, so th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com