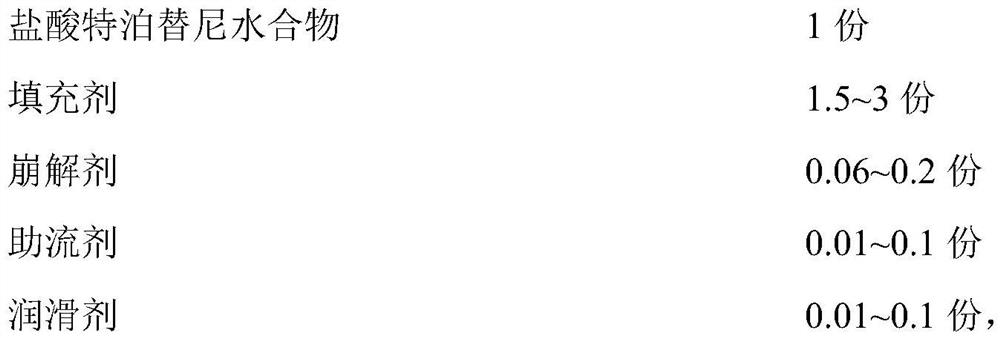Tepotinib tablet and preparation method thereof
A technology of tepotinib tablets and tablet cores, applied in the field of tepotinib tablets and its preparation, to achieve the effects of ensuring uniformity of mixing, reducing economic pressure, and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
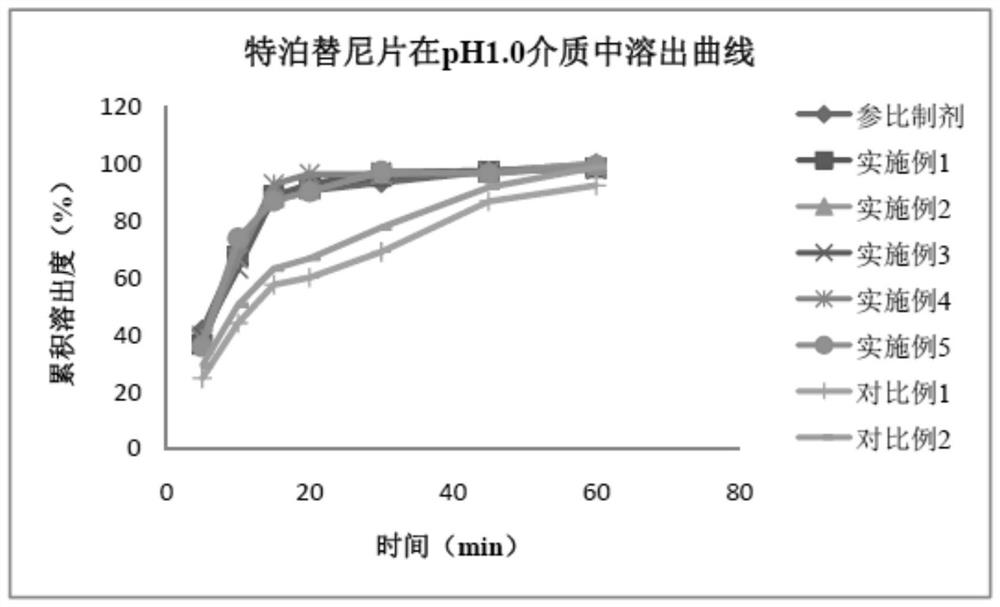
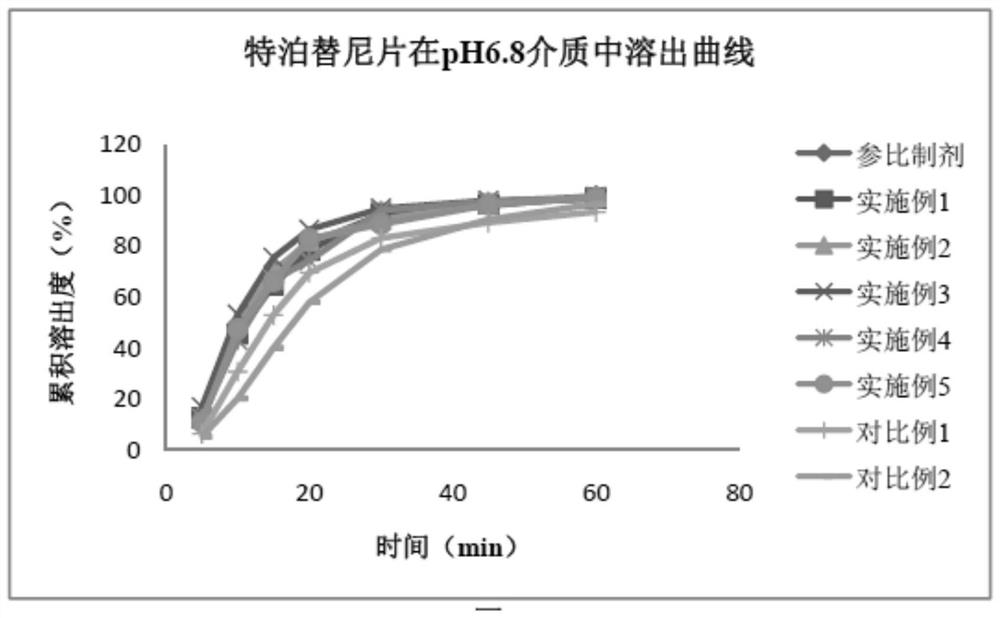
Image
Examples
Embodiment 1
[0041] A tepotinib tablet, the tablet specification is 250mg, consists of the following components:
[0042]
[0043] The dry granulation process is adopted, and the preparation method is as follows:
[0044] (1) 250g tepotinib hydrochloride hydrate, 329.76g mannitol, 103.57g microcrystalline cellulose, 16.57g crospovidone, 4.14g colloidal silicon dioxide and 2.07g magnesium stearate were mixed uniformly;
[0045] (2) performing dry granulation on the physical mixture obtained in step (1);
[0046] (3) Pass the granules obtained in step (2) through a 20-mesh sieve for granulation, add 103.57g microcrystalline cellulose, 16.57g crospovidone, 2.07g magnesium stearate and mix uniformly;
[0047] (4) Add the material obtained in step (3) into a tablet press to compress the tablet, and coat it with a coating solution until the weight gain is 3%, to obtain a tepotinib tablet.
Embodiment 2
[0049] A tepotinib tablet, the tablet specification is 250mg, consists of the following components:
[0050]
[0051] The dry granulation process is adopted, and the preparation method is as follows:
[0052] (1) 250g tepotinib hydrochloride hydrate, 329.76g mannitol, 103.57g microcrystalline cellulose, 16.57g crospovidone, 4.14g colloidal silicon dioxide and 2.07g magnesium stearate were mixed uniformly;
[0053] (2) performing dry granulation on the physical mixture obtained in step (1);
[0054] (3) Pass the granules obtained in step (2) through a 20-mesh sieve for granulation, add 103.57g microcrystalline cellulose, 16.57g crospovidone, 2.07g magnesium stearate and mix uniformly;
[0055] (4) Add the material obtained in step (3) into a tablet press to compress the tablet, and coat it with a coating solution until the weight gain is 5%, to obtain a tepotinib tablet.
Embodiment 3
[0057] A tepotinib tablet, the tablet specification is 250mg, consists of the following components:
[0058]
[0059] The dry granulation process is adopted, and the preparation method is as follows:
[0060] (1) Mix 250g tepotinib hydrochloride hydrate, 329.76g mannitol, 103.57g microcrystalline cellulose, 16.57g crospovidone, 4.14g colloidal silicon dioxide and 1.38g magnesium stearate;
[0061] (2) performing dry granulation on the physical mixture obtained in step (1);
[0062](3) Pass the granules obtained in step (2) through a 20-mesh sieve for granulation, add 103.57g microcrystalline cellulose, 16.57g crospovidone, 2..76g magnesium stearate and mix evenly;
[0063] (4) Add the material obtained in step (3) into a tablet press to compress the tablet, and coat it with a coating solution until the weight gain is 3%, to obtain a tepotinib tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com