Azithromycin dry suspension and preparation method thereof
A technology of azithromycin and dry suspension, which is applied in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., can solve problems such as the dissolution rate not reaching the ideal consistency, the gap between quality and curative effect, etc. , to achieve the effect of solving compliance problems and high consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
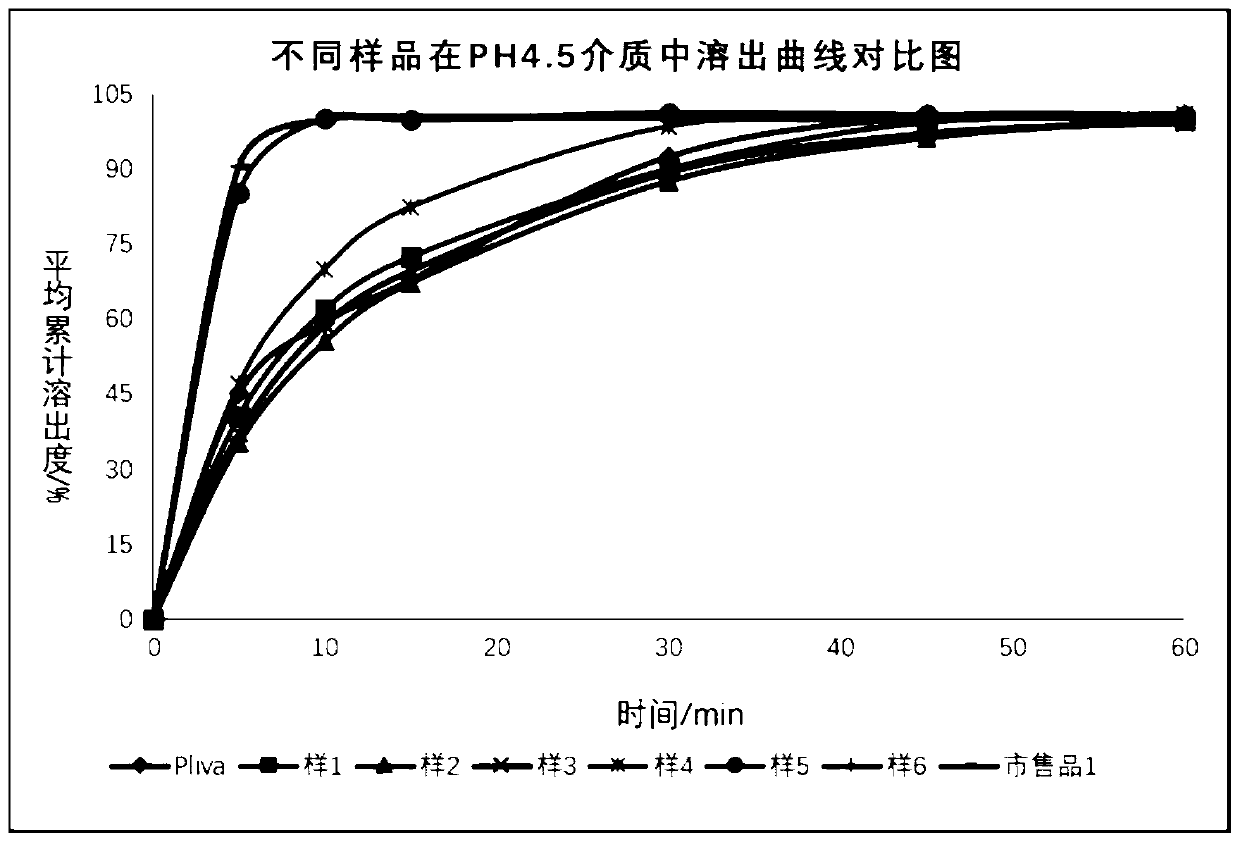
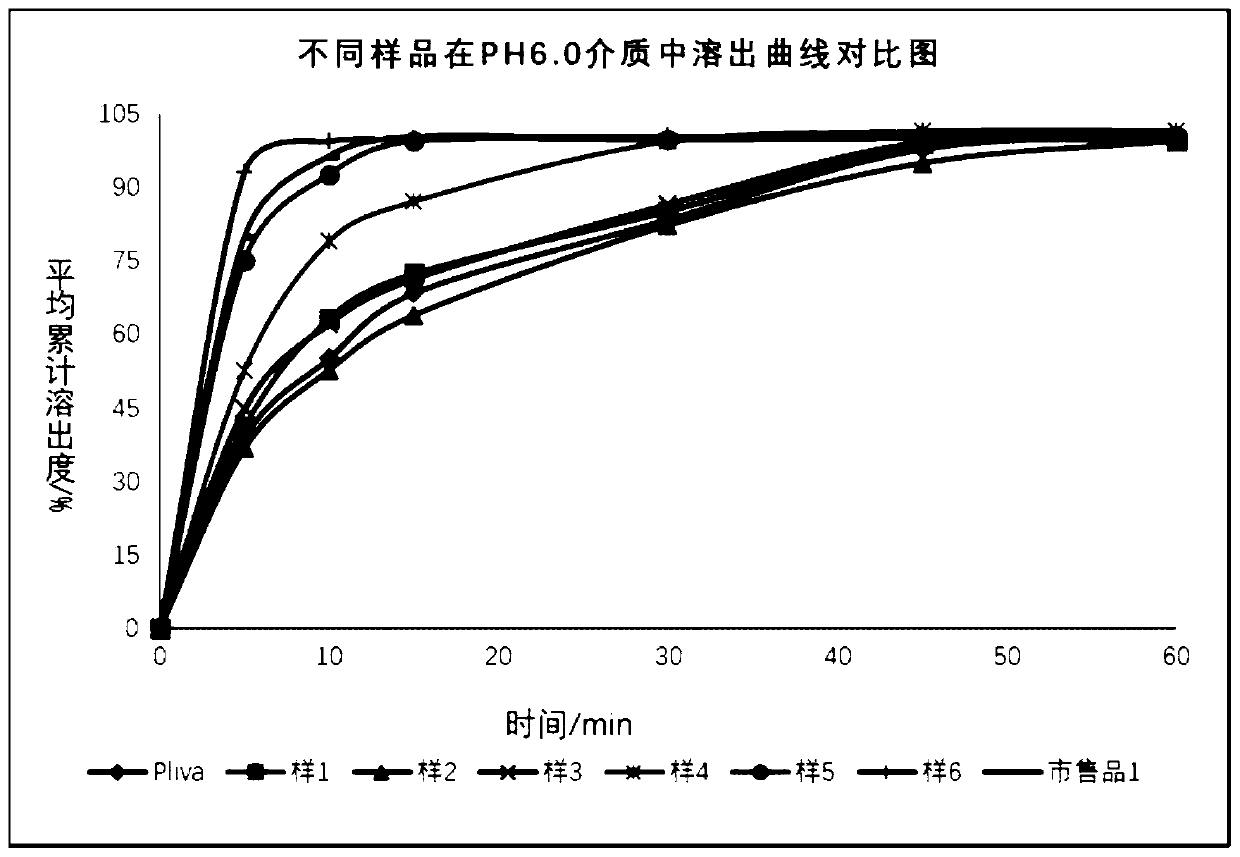
Examples
Embodiment 1
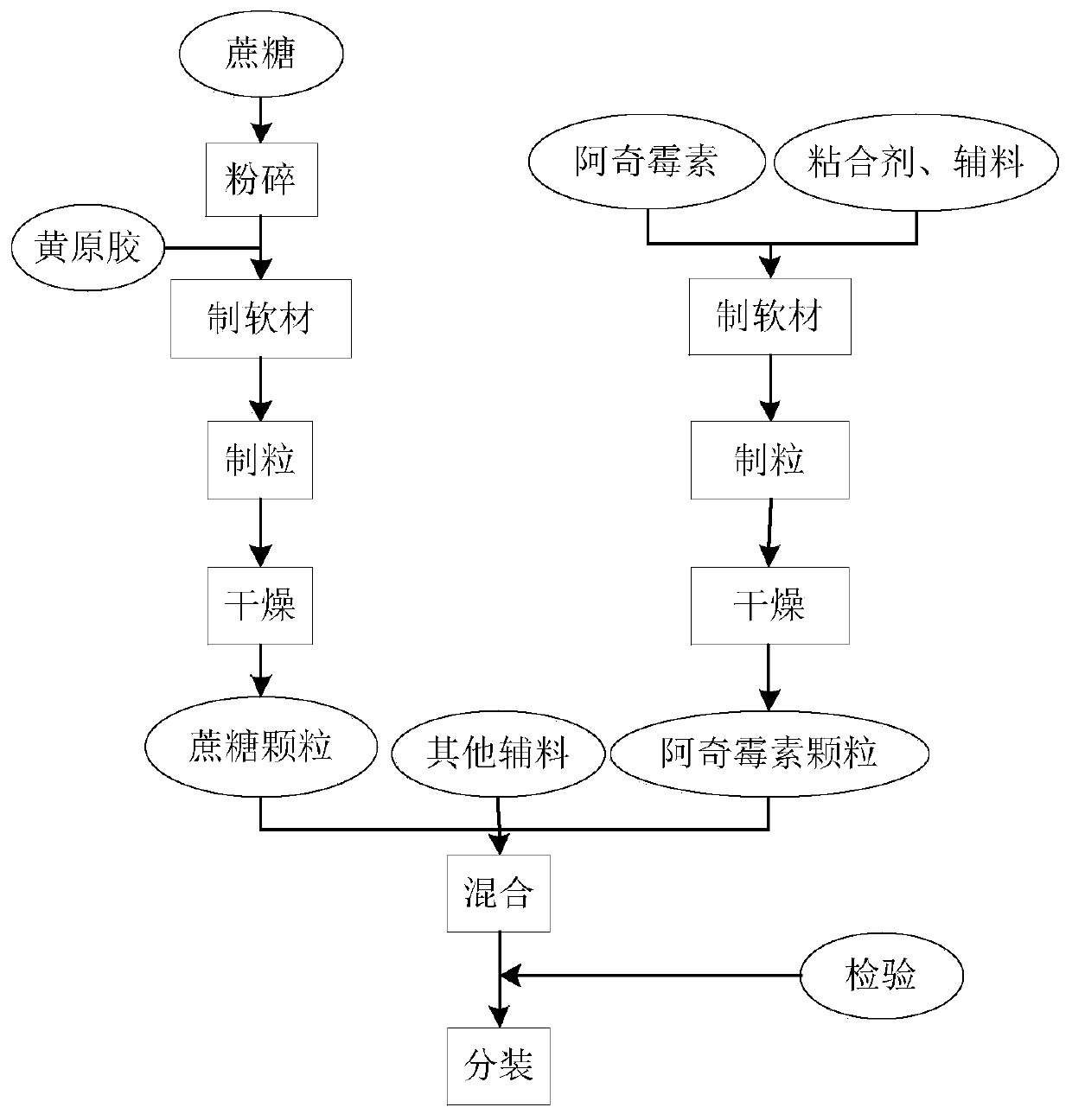
[0058] The present embodiment provides a kind of preparation method of azithromycin dry suspension, comprises the following steps (process flow chart sees figure 1):
[0059] 1) Sucrose is crushed to 60 meshes, azithromycin is crushed to 100 meshes, trisodium phosphate anhydrous, hydroxypropyl cellulose, xanthan gum and essence are crushed to 40 meshes for later use.
[0060] 2) Weigh 10kg of azithromycin, 388kg of sucrose, 1.5kg of anhydrous trisodium phosphate, 1kg of hydroxypropyl cellulose, 1kg of xanthan gum, 1.5kg of cherry powder essence, 0.24kg of banana powder essence, and 2kg of silicon dioxide.
[0061] 3) Adhesive preparation: prepare an aqueous solution by adding 20 g of hydroxypropyl cellulose and 1000 ml of purified water, and set aside.
[0062] 4) Preparation of sucrose granules: Add 388kg of sucrose powder and 1kg of xanthan gum to a high-speed wet granulator and mix for 5 minutes, add 11.6kg of purified water to make a soft material, shake it through 24 mes...
Embodiment 2
[0067] The present embodiment provides a kind of preparation method of azithromycin dry suspension, comprising the following steps:
[0068] 1) Sucrose is crushed to 60 meshes, azithromycin is crushed to 100 meshes, trisodium phosphate anhydrous, hydroxypropyl cellulose, xanthan gum and essence are crushed to 40 meshes for later use.
[0069] 2) Weigh 10kg of azithromycin, 388kg of sucrose, 1.5kg of anhydrous trisodium phosphate, 1kg of hydroxypropyl cellulose, 1kg of xanthan gum, 1.5kg of cherry powder essence, 0.24kg of banana powder essence, and 2kg of silicon dioxide.
[0070] 3) Adhesive preparation: prepare an aqueous solution by adding 30 g of hydroxypropyl cellulose and 1000 ml of purified water, and set aside.
[0071] 4) Preparation of sucrose granules: Add 388kg of sucrose powder and 1kg of xanthan gum to a high-speed wet granulator and mix for 5 minutes, add 11.6kg of purified water to make a soft material, shake it through 24 mesh, and dry at 60°C for 2 hours to o...
Embodiment 3
[0076] The present embodiment provides a kind of preparation method of azithromycin dry suspension, comprising the following steps:
[0077] 1) Sucrose is crushed to 60 meshes, azithromycin is crushed to 100 meshes, trisodium phosphate anhydrous, hydroxypropyl cellulose, xanthan gum and essence are crushed to 40 meshes for later use.
[0078] 2) Weigh 10kg of azithromycin, 388kg of sucrose, 1.5kg of anhydrous trisodium phosphate, 1kg of hydroxypropyl cellulose, 1kg of xanthan gum, 1.5kg of cherry powder essence, 0.24kg of banana powder essence, and 2kg of silicon dioxide.
[0079] 3) Adhesive preparation: prepare an aqueous solution by adding 20 g of hydroxypropyl cellulose and 1000 ml of purified water, and set aside.
[0080] 4) Preparation of sucrose granules: Add 388kg of sucrose powder and 1kg of xanthan gum to a high-speed wet granulator and mix for 5 minutes, add 13.5kg of purified water to make a soft material, shake it through 24 mesh, and dry at 60°C for 2 hours to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com