Simultaneous measurement of ligustrazine and aspirin in blood plasma by LC-MS
An LC-MS and aspirin technology, applied in the field of medicine, can solve the problems of no simultaneous detection, reduce the bleeding tendency of aspirin, poor patient compliance, etc., and achieve the effects of simple and easy operation, accurate detection concentration, and low detection concentration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] The following examples are further illustrations of the present invention, but the present invention is not limited to the following examples.
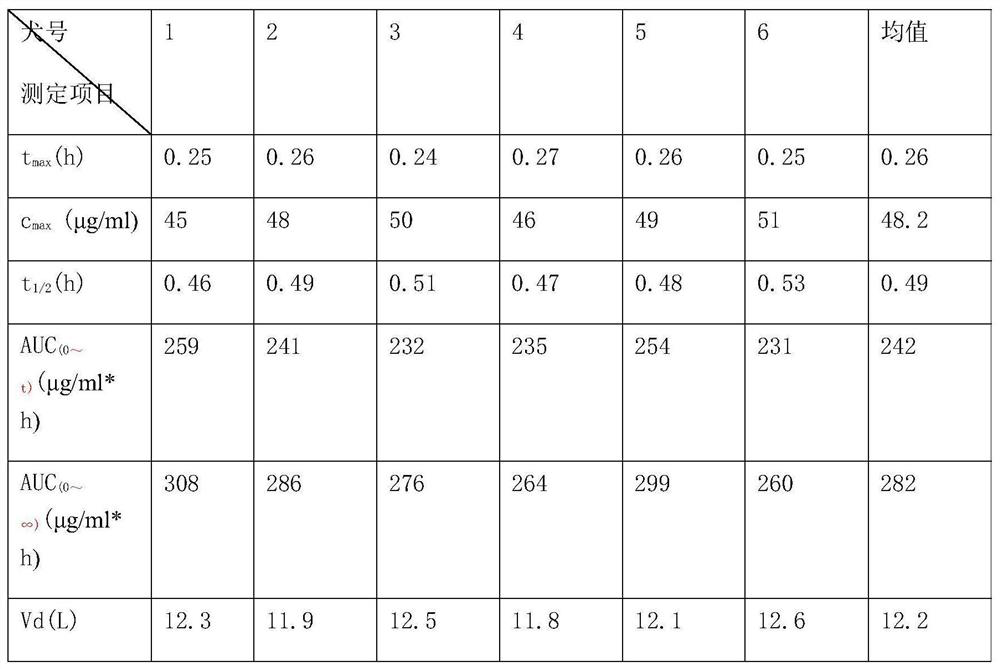
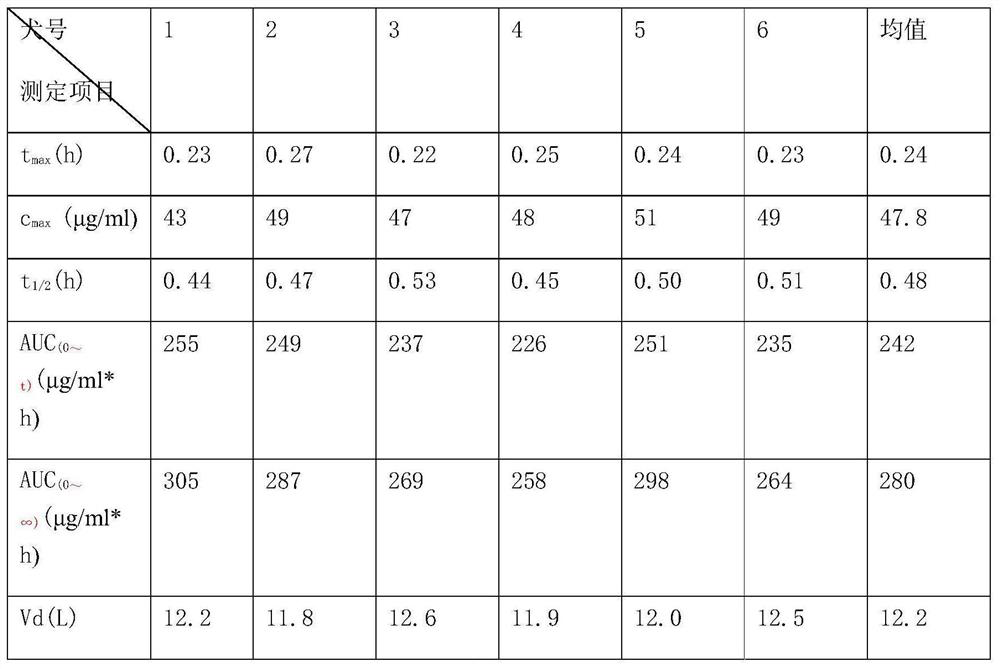
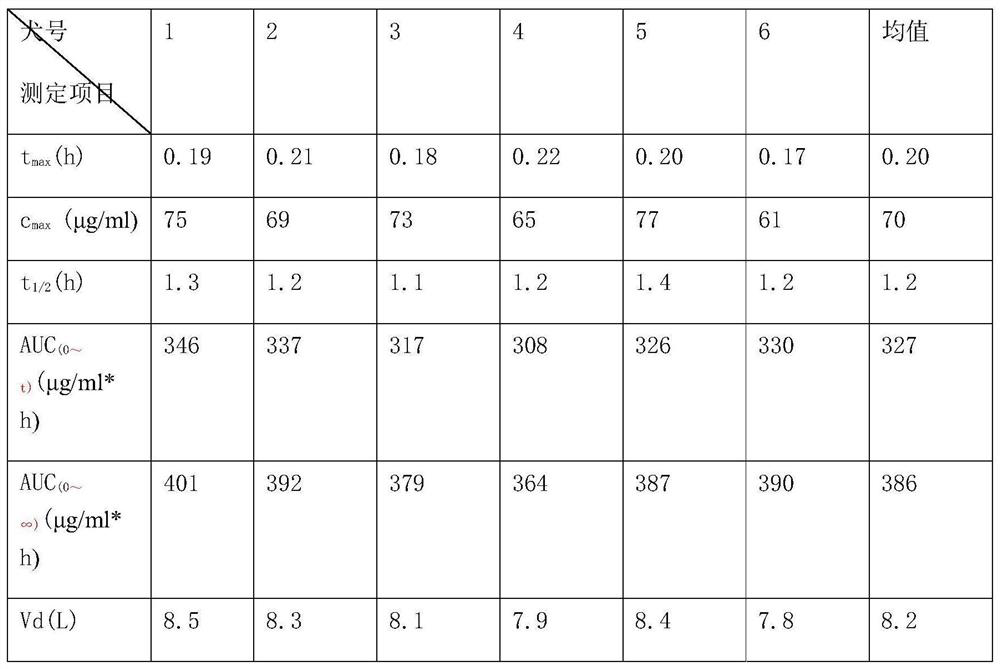
[0016] Simultaneous determination of ligustrazine and aspirin in plasma by LC-MS, characterized in that it includes a chromatographic column filled with octadecyl-bonded silica gel, the liquid phase flow rate is 0.6ml / min-1.4ml / min, and the mobile phase is composed of methanol and formic acid The aqueous solution is prepared according to certain equipment, and the LC-MS isocratic elution method is used to separate and analyze Ligustrazine, aspirin and their metabolites in the plasma sample after treatment. The proportion of methanol in the mobile phase is usually 40% to 90%. solution system, but the solution of methanol / 0.1% formic acid aqueous solution (80:20) is usually used. The mixed solution of methanol and formic acid water means that the pH value of methanol, formic acid and pure water is between 2-3 The concentration of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com