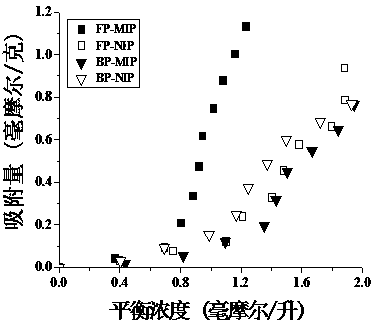
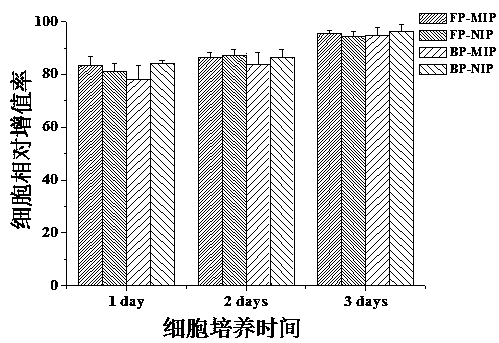
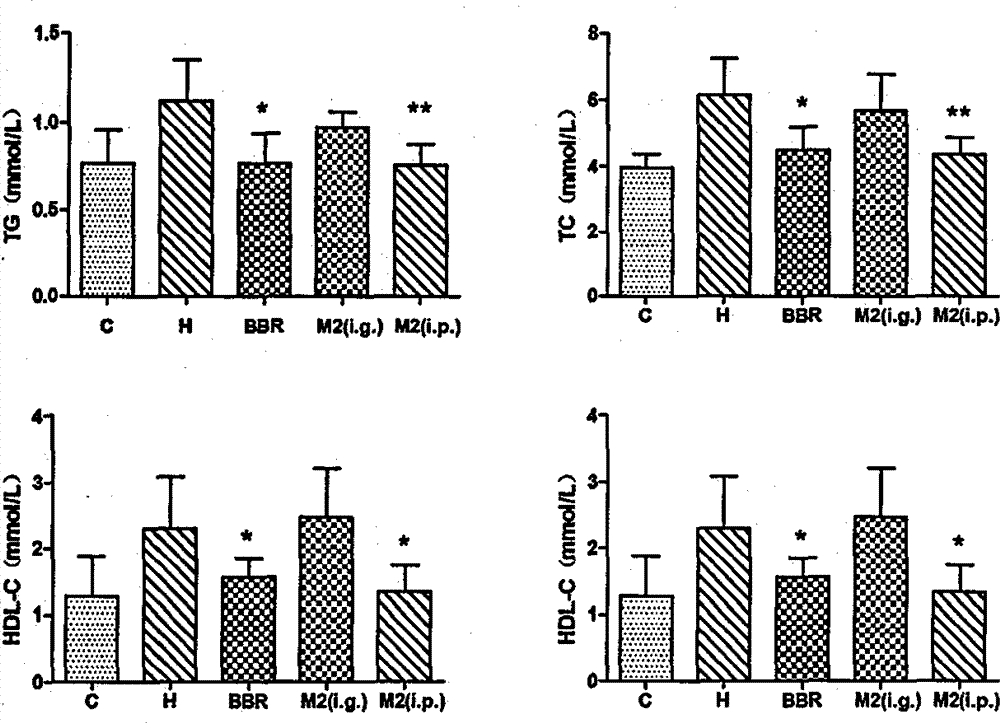
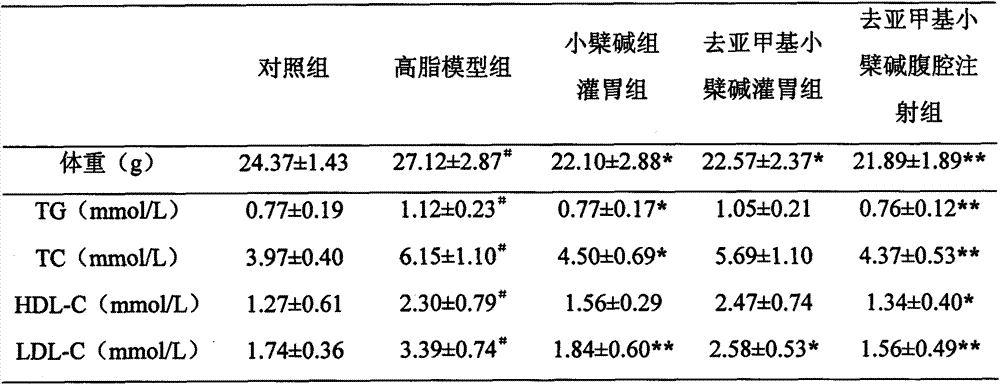
Patents
Literature
171 results about "In vivo metabolism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compound vegetable healthcare product and method for preparing same
InactiveCN102228242ATo promote metabolismPromote peristalsisFood preparationBiotechnologyFood additive
The invention relates to a nutrient healthcare product and a method for preparing the same, in particular to a compound vegetable healthcare product and a method for preparing the same. The compound vegetable nutrient powder of the invention consists of the following components in part by weight: 0.1 to 100 parts of dried vegetable powder, 0 to 10 parts of alga-type plant regulating powder, 0 to 96 parts of auxiliary component, 0 to 5 parts of natural flavoring agent and 0 to 5 parts of food additive. The preparation method adopts a simple production process and is suitable for industrialized production, and the product prepared by the method is rich in nutrition and stable in quality, meets the demands of people on various nutrients, regulates the intestines and stomach, improves enterogastric peristalsis, enhances in vivo metabolism of sulfur-contained substances, avoids the adverse effects of human body caused by malnutrition and unbalanced nutrition and prevents and helps to treat diseases such as ulcerative stomatitis, diabetes and constipation. The product has also the advantages of convenience for carrying and long storage period.
Owner:肖天存
Composite plant enzyme containing probiotics and application of composite plant enzyme
ActiveCN103876155APromote body metabolismWide range of conditionsFood ingredient functionsFood preparationBiotechnologyLactase
The invention discloses a composite plant enzyme containing probiotics and an application of the composite plant enzyme. The composite plant enzyme comprises amylase, lipase, protease, cellulase, beta-dextranase, xylanase and lactase. The composite plant enzyme is obtained by discontinuously feeding materials and fermenting for a plurality of periods, selecting a plurality of types of fruits and vegetables, and edible and medical plants as fermentation substrates and taking a plurality of types of probiotics / yeasts as fermentation inoculation substances through a plurality of grades of deep fermentation. The composite plant enzyme contains seven enzymes needed by a human body at the same time so that the plant enzyme has the synergistic effects of promoting in-vivo metabolism and decomposing toxic substances; the plant enzyme contains a high-activity compound enzyme and has a wide acting condition, good adaptability, and good stability, acid-base resistance and high-temperature resistance; the plant enzyme can effectively and rapidly improve the biochemical reaction speed and can be used as a raw material to be applied to foods and chemicals for daily use.
Owner:田雷
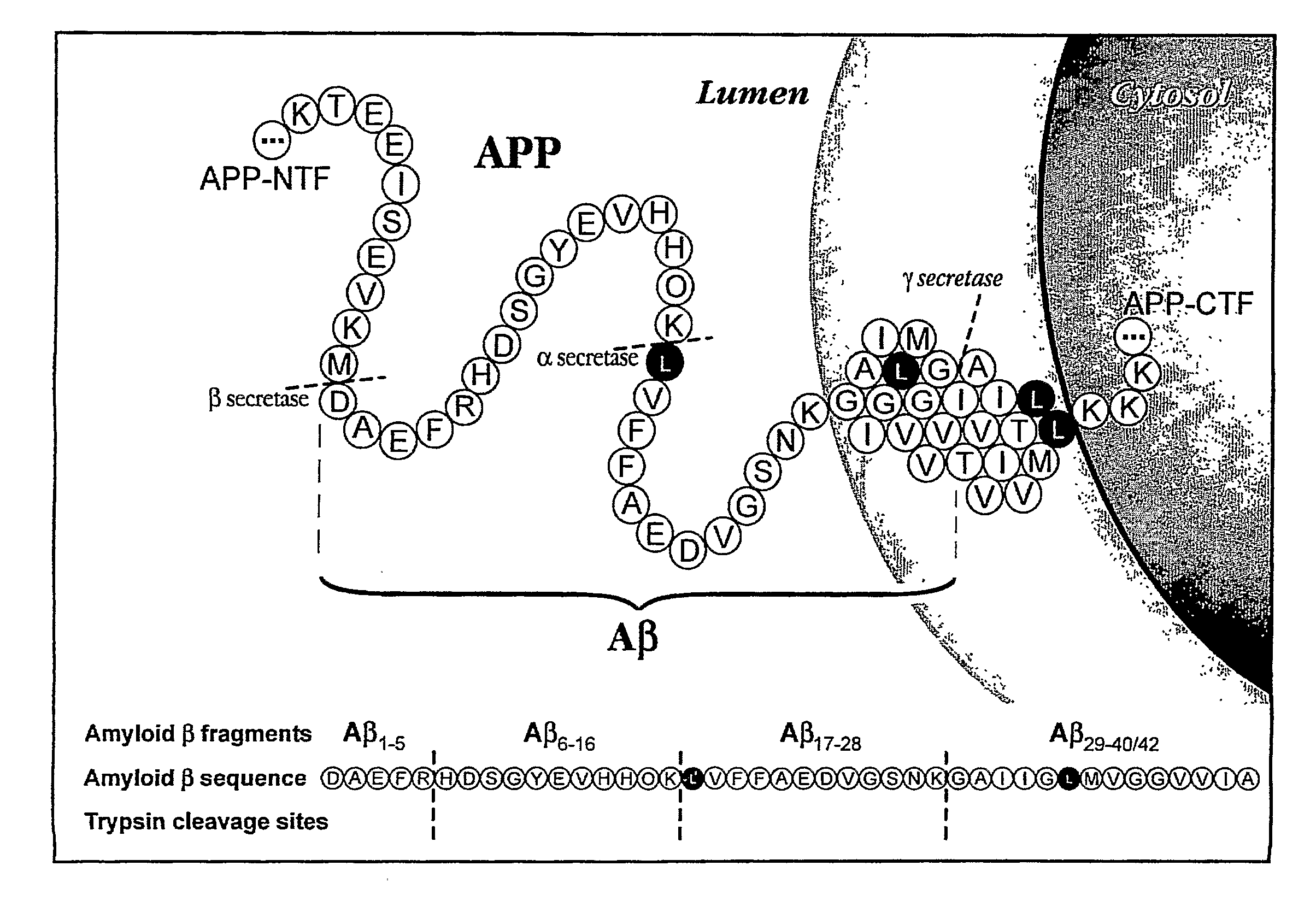
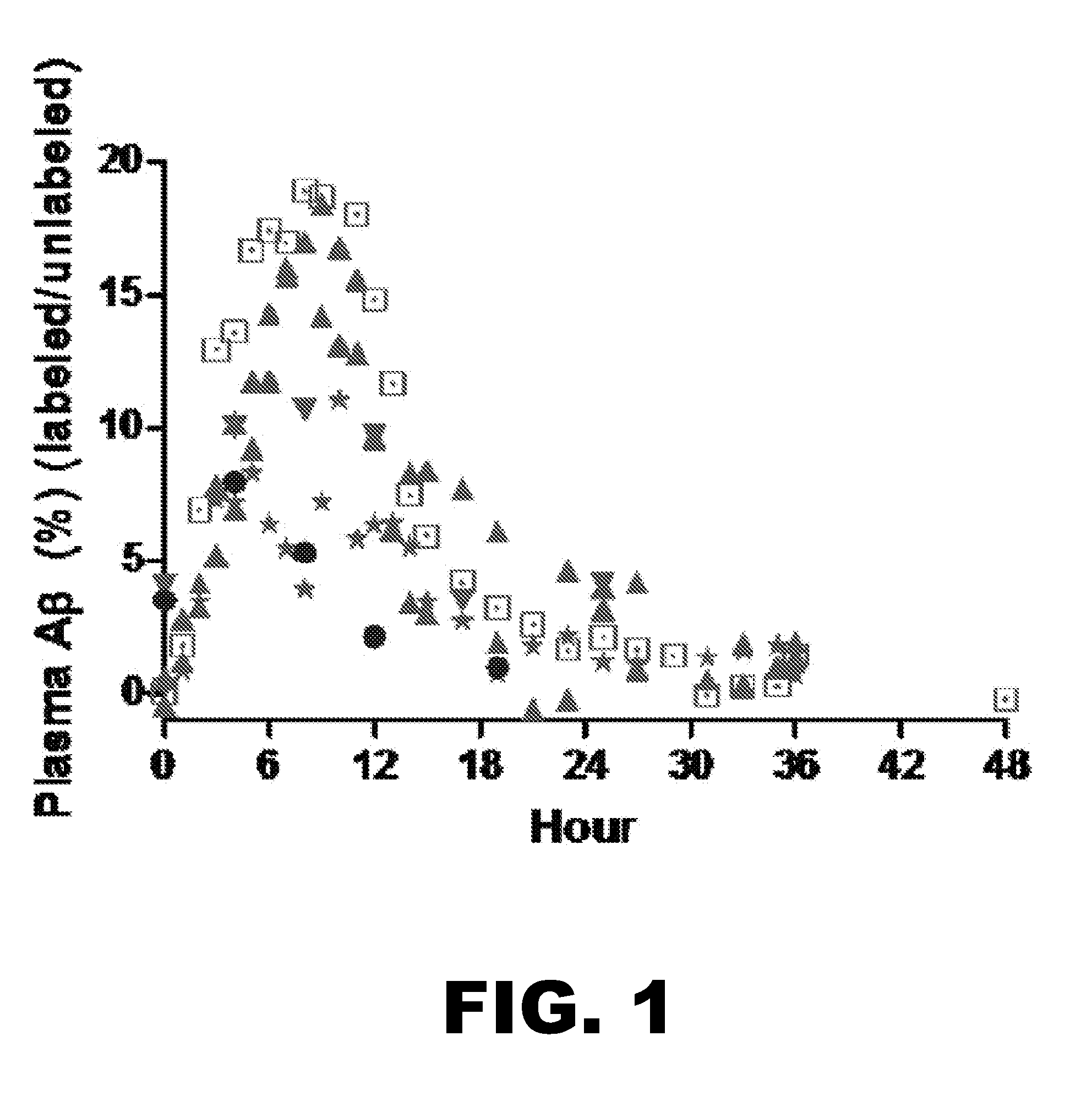
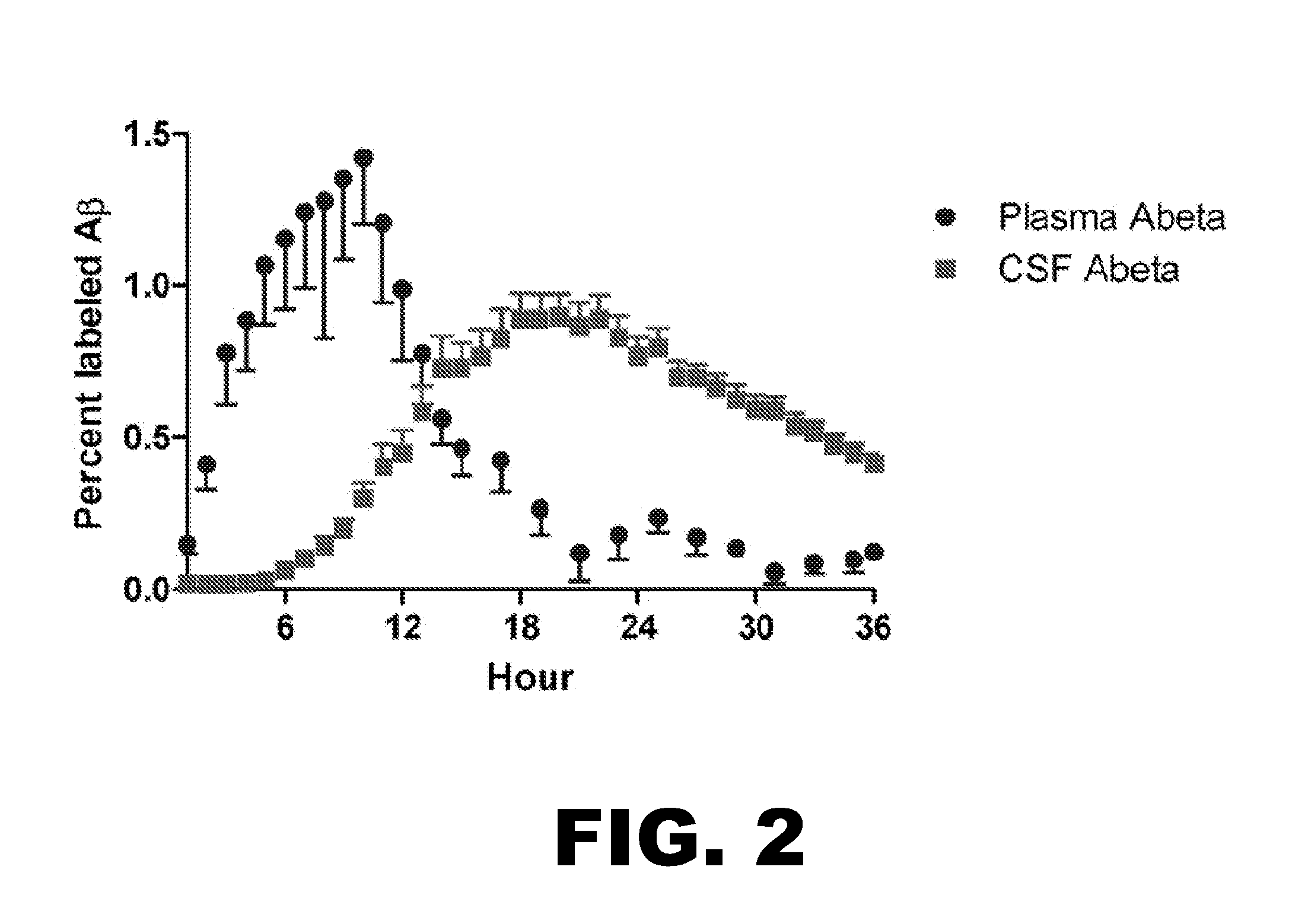
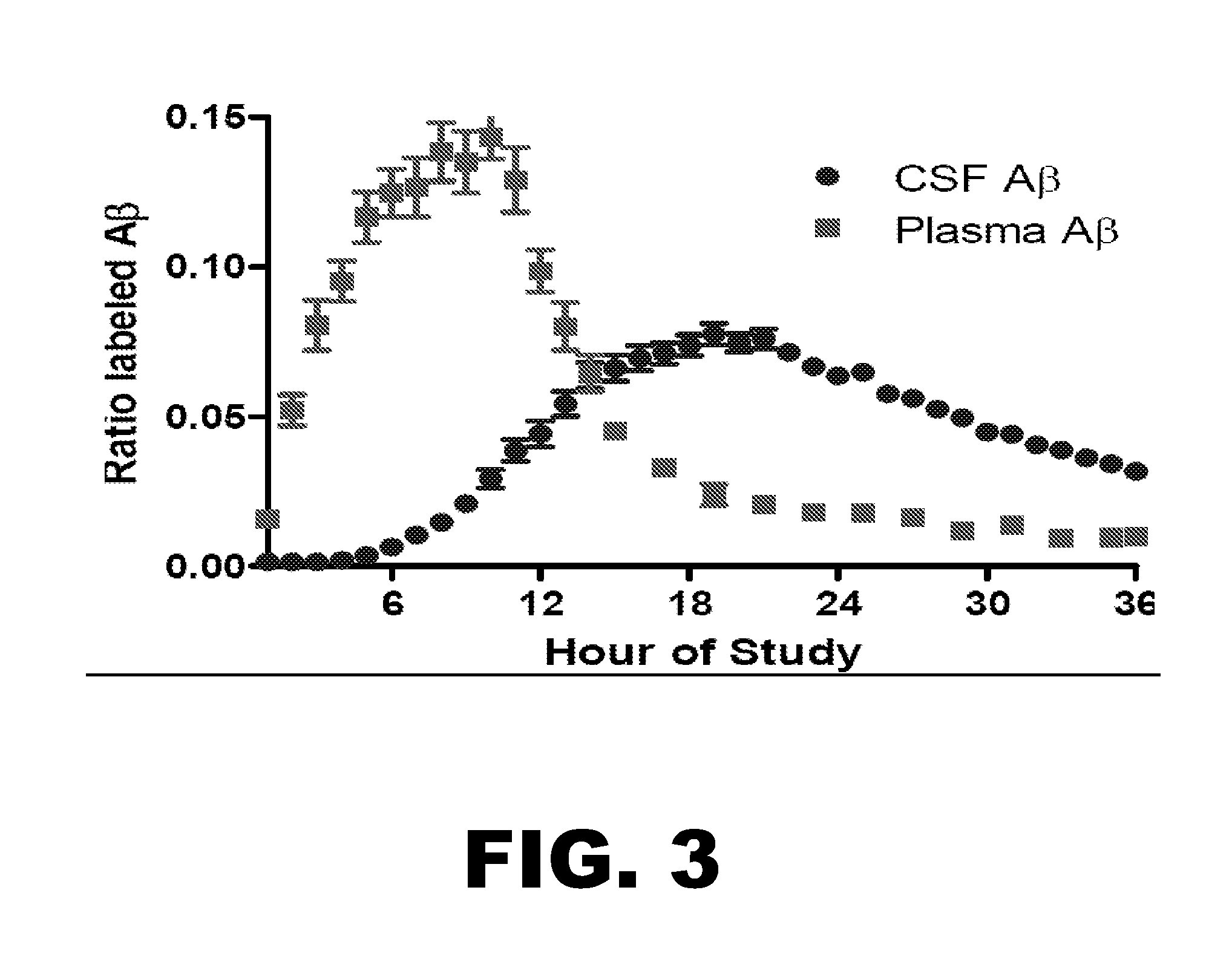
Methods for Measuring the Metabolism of Neurally Dervied Biomolecules in Vivo
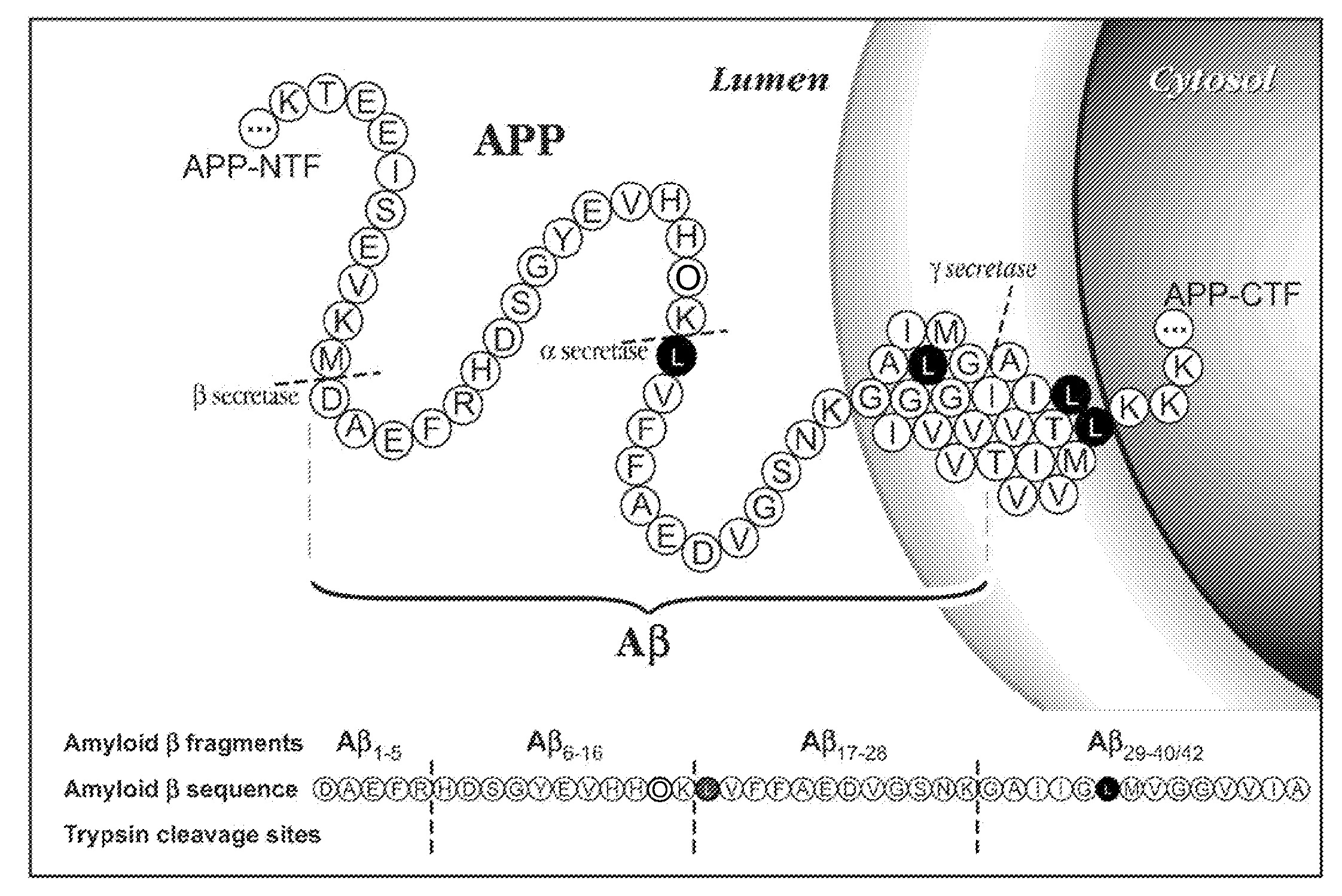
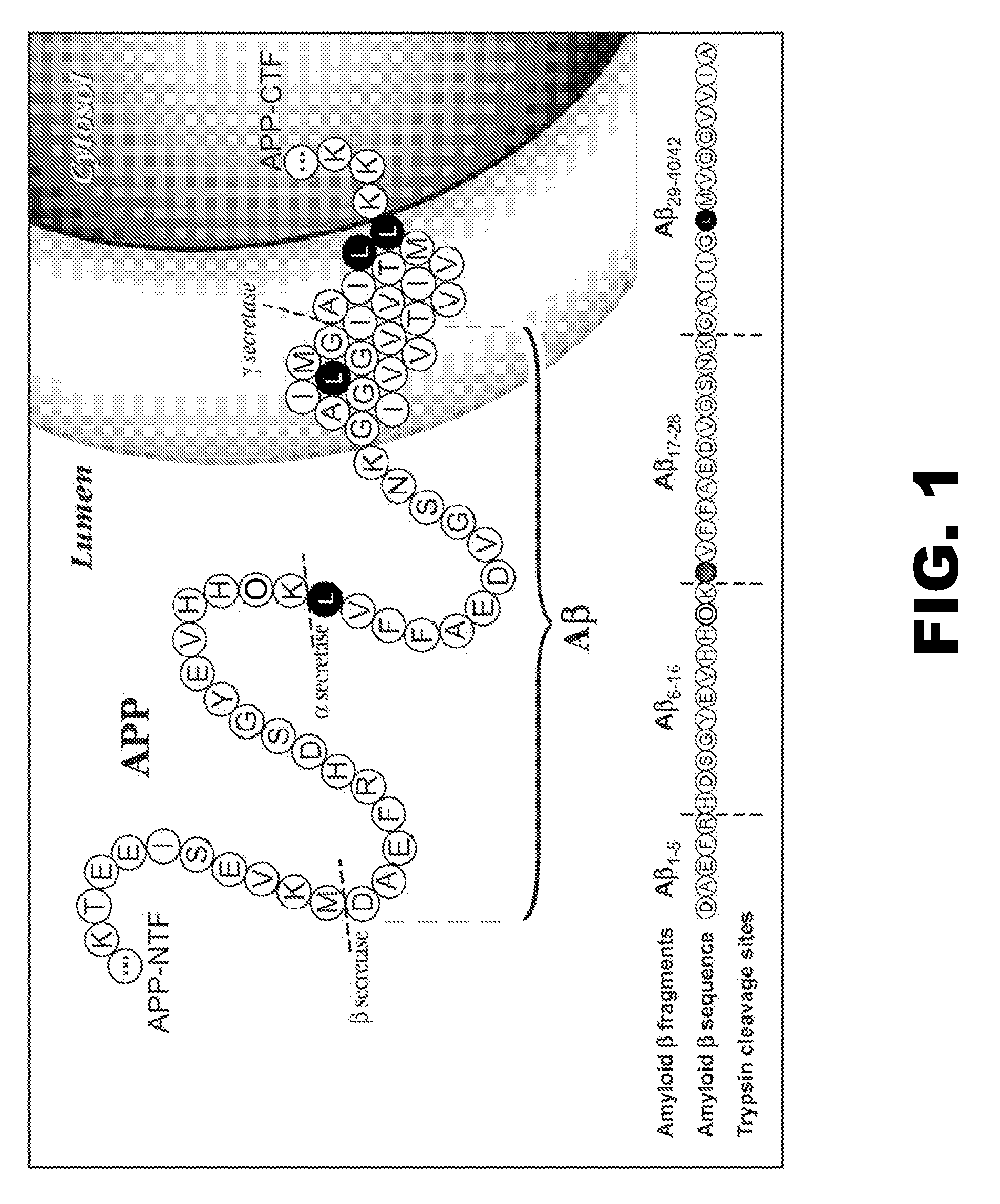
The present invention relates to methods of diagnosing, monitoring, and assessing treatment effects for neurological and neurodegenerative diseases and disorders, such as Alzheimer's Disease, early in the course of clinical disease or prior to the onset of brain damage and clinical symptoms. Methods of measuring the in vivo metabolism of biomolecules produced in the CNS in a subject are provided.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Methods for measuring the metabolism of CNS derived biomolecules in vivo
InactiveUS20090142766A1Microbiological testing/measurementAssay labelsNervous systemBiological organism
The present invention provides methods for measuring the metabolism of a central nervous system derived biomolecule implicated in a neurological and neurodegenerative disease or disorder. In particular, the method comprises measuring the in vivo metabolism of the biomolecule in the central nervous system of a subject. Also provided is a method for determining whether a therapeutic agent affects the in vivo metabolism of a central nervous system derived biomolecule.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Paramagnetic metal complex functionalized fluorogold nano-cluster magnetic resonance and fluorescence imaging contrast agent
InactiveCN102366632AImprove spatial resolutionHigh sensitivitySerum albuminIn-vivo testing preparationsFluorescenceBovine serum albumin
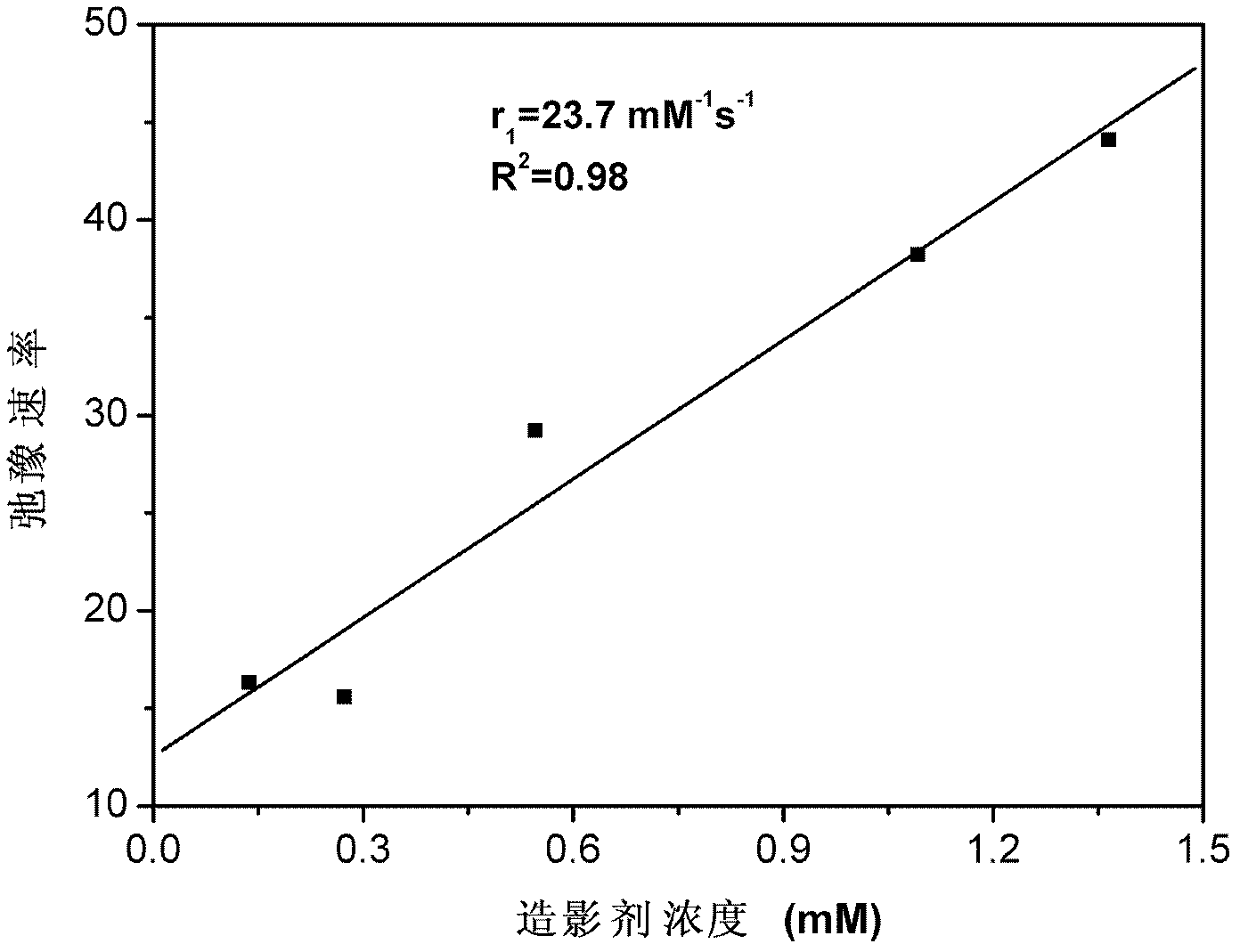
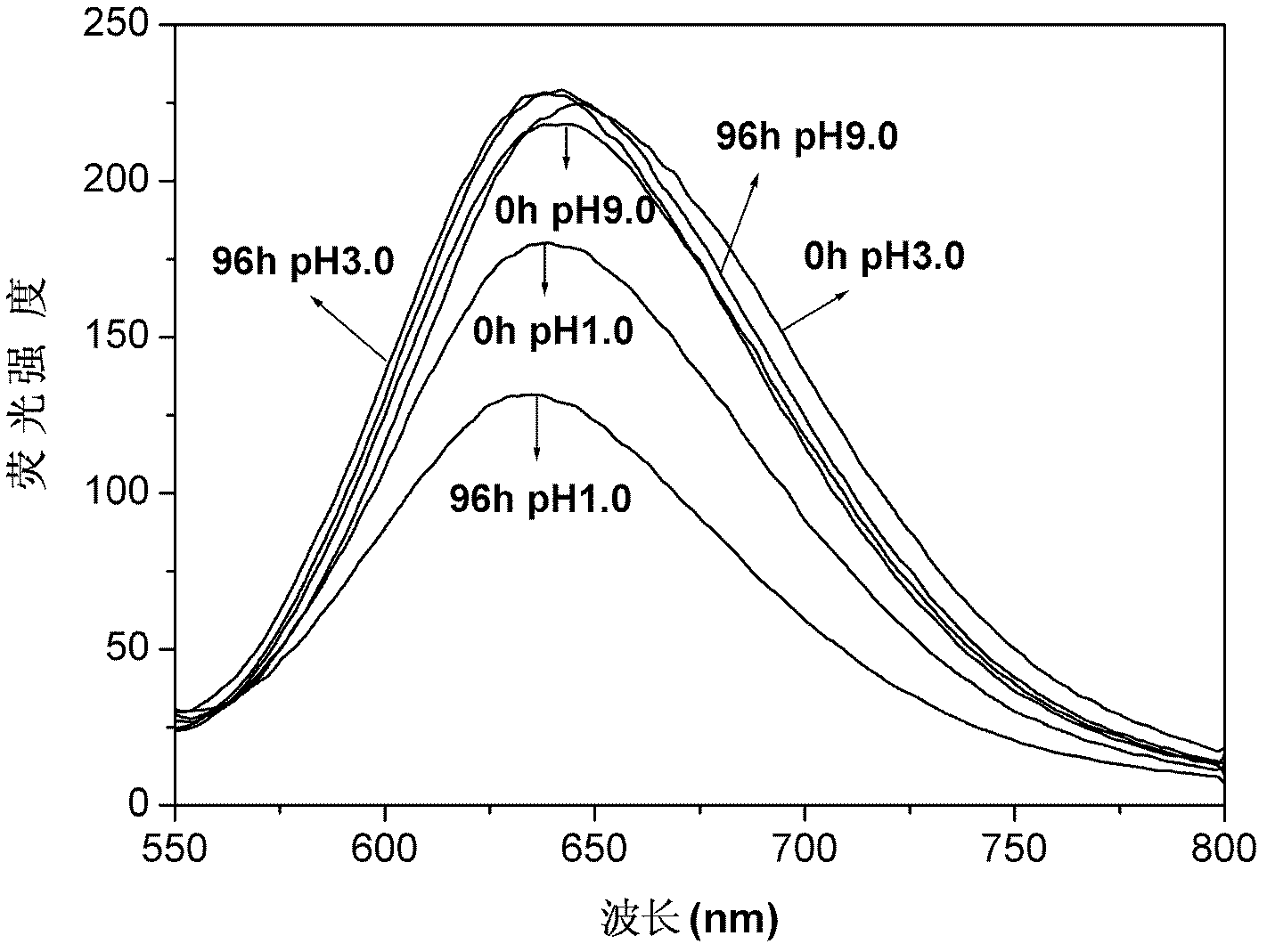
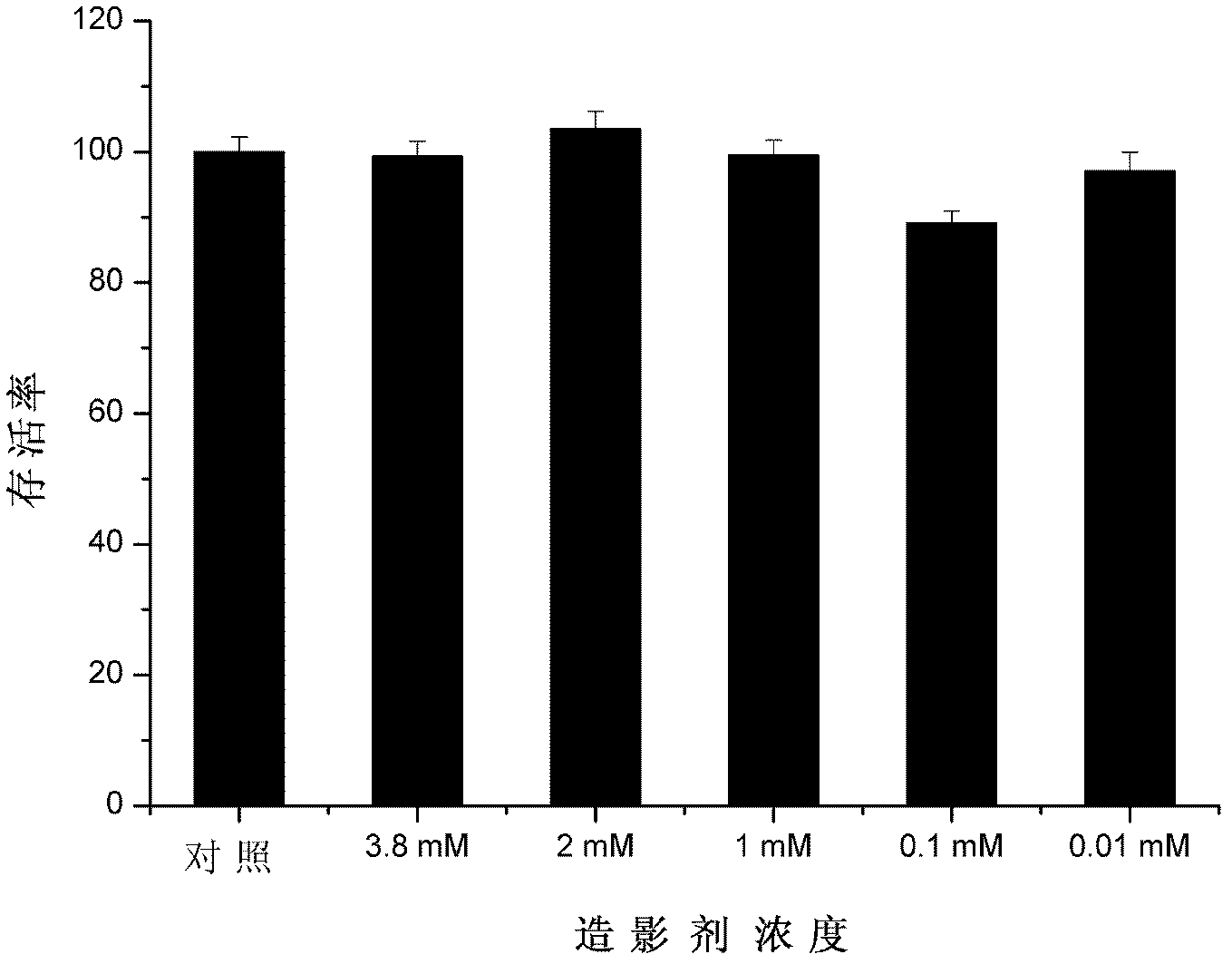
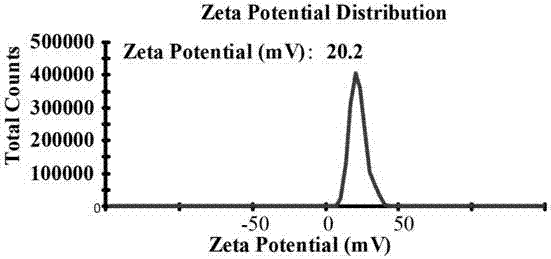
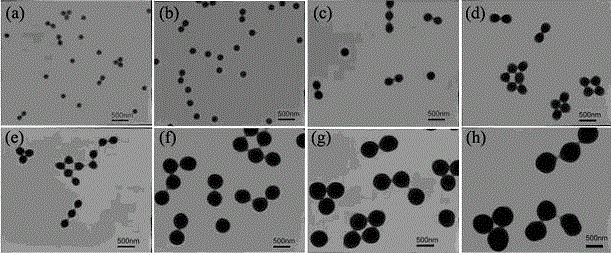
The invention provides a paramagnetic metal complex functionalized fluorogold nano-cluster magnetic resonance and fluorescence imaging contrast agent. The invention relates to the field of magnetic resonance imaging and fluorescence imaging contrast agents. With the contrast agent provided by the invention, problems of existing contrast agents of low relaxation efficiency, high toxicity, low tissue organ selectivity and fast in-vivo metabolism can be solved. According to the invention, ethylenediaminetetraacetic acid (EDTA) or diethylene triaminepentaacetic acid (DTPA) are connected to folic-acid-modified bovine serum albumin fluorogold nano-clusters through amide bonds; and EDTA or DTPA is respectively coordinated with paramagnetic metal ions, such that the complex is obtained. With the contrast agent provided by the invention, the relaxation efficiency can reach 23.7mM<-1>*s<-1>. The contrast agent has an advantage of low toxicity. According to the invention, high spatial resolution of magnetic resonance imaging and high sensitivity of fluorescence imaging are combined. Therefore, the contrast agent provided by the invention can be used in magnetic resonance imaging and fluorescence imaging.
Owner:CHANGCHUN UNIV OF TECH
Carrier-free co-assembled tumor targeting anti-cancer nano medicine as well as preparation method and application thereof
InactiveCN107158014AGood tumor treatmentSolve complexityPowder deliveryOrganic active ingredientsTumor targetAptamer
The invention discloses a carrier-free co-assembled tumor targeting anti-cancer nano medicine as well as a preparation method and application thereof. The carrier-free dual anti-cancer nano medicine is prepared from a hydrophobic medicine, namely ursolic acid, together with board-spectrum anti-tumor medicines such as doxorubicin in water through co-assembling, in addition, a fluorescence labeling nucleic acid aptamer, a molecular target, an antibody or polypeptide and the like with tumor targeting functions are adsorbed to the surface of the medicine through mutual electrostatic functions, then the carrier-free co-assembled tumor targeting anti-cancer nano medicine with tumor targeting and tumor microenvironment response is prepared, a synergic anti-tumor function is achieved, diagnosis and treatment integration is achieved, particularly the medicine has outstanding functions in preventing tumor transfer, and more importantly the problems that a conventional nano carrier is complex in system, indefinite in in-vivo metabolism and the like are solved.
Owner:FUZHOU UNIV
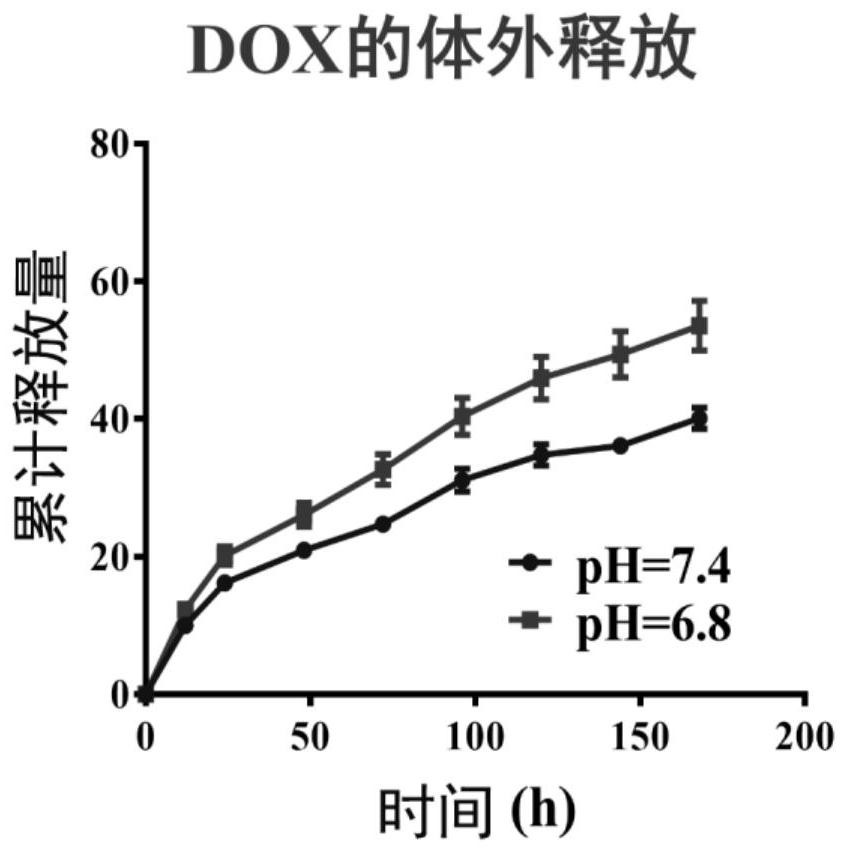
Nano-hydrogel with oxidation-reduction/pH double-stimulation responsiveness and preparation method and application thereof
InactiveCN102973488AQuick releaseEfficient releaseOrganic active ingredientsPharmaceutical delivery mechanismEthyl groupFolate targeting
The invention belongs to the technical field of biological medicine, and in particular relates to a nano-hydrogel with oxidation-reduction / pH double-stimulation responsiveness and a preparation method and application thereof. The preparation method comprises the following steps of: adding a polymerized monomer and a crosslinking agent into a solvent to perform a polymerization reaction, and distilling the deposits to remove a half of the solvent; removing the solvent and the unreacted monomer, and drying to obtain polymer gel particles; or further modifying different functional groups such as on-belt amino groups and the like, and performing a reaction on 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) / N-hydroxysuccinimide (NHS) and amino-folic acid to obtain folate-targeted nano-hydrogel particles. The nano-hydrogel is in morphologically regular spherical shape, uniform in size and controllable in particle size, has oxidation-reduction and pH double-stimulation responsiveness, good colloidal stability and dispersity, is used as a drug carrier, and can be easily discharged out of a body through the kidney during subsequent in vivo metabolism.
Owner:FUDAN UNIV
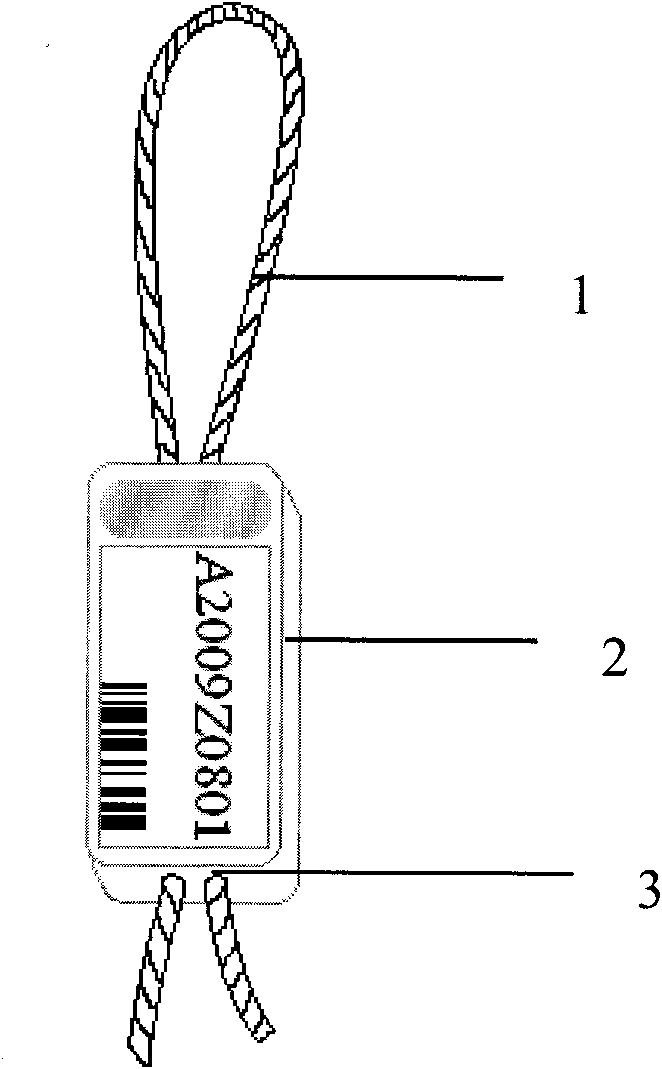
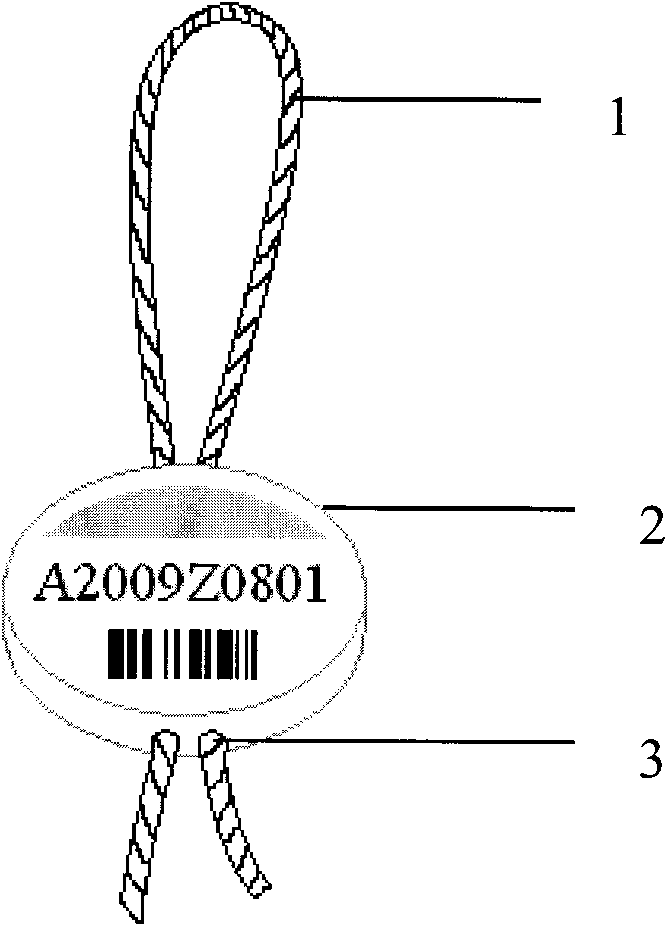
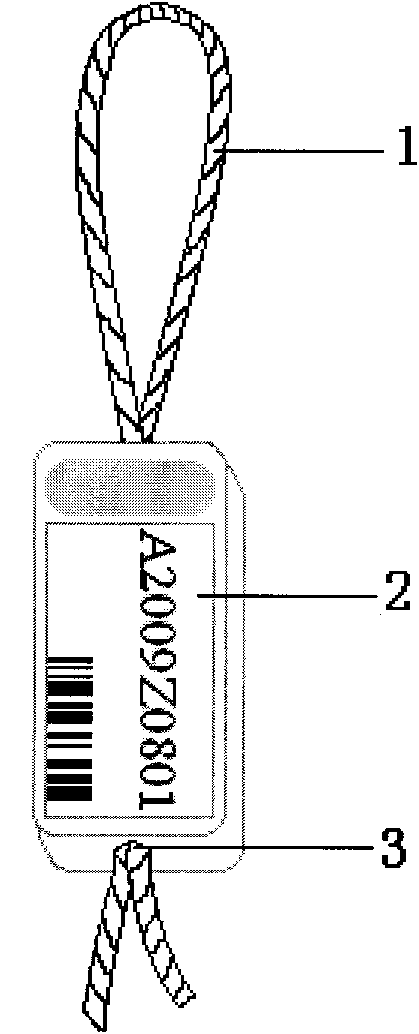
Method for labeling shrimps and shrimp label
InactiveCN101773086ADoes not affect food intakeDoes not affect movementStampsClimate change adaptationSide effectShrimp
The invention discloses a method for labeling shrimps, particularly for fixing a shrimp eyestalk label to a shrimp body through a shrimp eyestalk. The label comprises a label string anchored and sleeved on the shrimp eyestalk, and a label body loaded with a mark, wherein the label string connected to the label body constitutes a stretchable closed ring on one side of the label body; and a central hole penetrating the label body is formed on the label body, so that the label string can be in non-fixed connection with the label body via the central hole to achieve the stretchability. When in use, the eyestalk on one side of the shrimp is lifted up, so that the ring-shaped part of the label string can be sleeved at the middle part of the eyestalk; and the label string is properly pulled, and the two free ends of the label string are clamped and burned by a hot tip-shaped body, so that the tail end of the label string can be sealed up and aligned with the label body. The shrimp label has the advantages of small size, light weight and no toxicity or side effects, thereby causing no impact on the ingestion and movement of the shrimps; the shrimp label is corrosion-resistant, high-pressure resistant and non-fading, so the shrimp label is particularly compatible to the life habit of aquatic organisms; and by using the shrimp eyestalk as the loading part for the label, the label is no longer physiologically effected by shrimp molting and in-vivo metabolism, so the shrimp label is durable and reliable.
Owner:SOUTH CHINA SEA INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Nutrients replenisher for replenishing folic acid and vitamin B12 and preparation method thereof
InactiveCN101176734AMeets the daily recommended amountOrganic active ingredientsMetabolism disorderNutrition supplementationNutrition
The invention relates to a nutriment supplement and a preparation method for the small dosage folic acid and vitamin B12. The folic acid cannot be synthesized by the human body, which must rely on the exogenetic supply. The folic acid has close relation with the vitamin B12 on the in vivo metabolism and the function. Although a plurality of preparations containing the folic acid are seld in the market, most of which are the preparation comprising the multivitamins, the calcium iron and other nutrient preparations or the sole preparation comprising the folic acid; the preparation only comprising the folic acid and the vitamin B12 contains the big dosage folic acid and the big dosage folic acid is not suitable for the pregnant women and the crowd lacking of folic acid. The invention is characterized in comprising the folic acid, the vitamin B12 and the medicine carrier according to the weight account matching 10 to 400: 1: 400 to 4000; the nutriment supplement is made into a capsule (tablet) comprising the folic acid 100 to 400 Mug, the vitamin B12 1 to 10 Mug according to the conventional method in the pharmaceutics. The invention has an advantage of capability for supplying the reasonable nutrition supplement to the people lacking of folic acid.
Owner:江西省药物研究所
Oil-in-water type nanometer grassleaved sweetflag oil emulsion oral liquid and its prepn process
InactiveCN1931293AHigh thermodynamic stabilityEasy to operateNervous disorderRespiratory disorderOil emulsionDistilled water
The oil-in-water nanometer grassleaved sweetflag oil emulsion oral liquid consists of surfactant, oil, grassleaved sweetflag oil and distilled water. Its preparation process includes the following steps: weighing surfactant with or without co-surfactant; calculating HLB value and selecting oil phase for reaching emulsifying HLB value near that of the surfactant phase; changing the ratio between the surfactant phase and the oil phase regularly; adding grassleaved sweetflag oil into surfactant through stirring; adding distilled water slowly at 20-25 deg.c to form clear and flowing O / W type nanometer emulsion liquid. The nanometer grassleaved sweetflag oil emulsion preparing process results in raised medicine stability, raised bioavailability, delayed in vivo metabolism time, reduced supplementary material amount, lowered production process and wide medicine market foreground.
Owner:NORTHWEST A & F UNIV
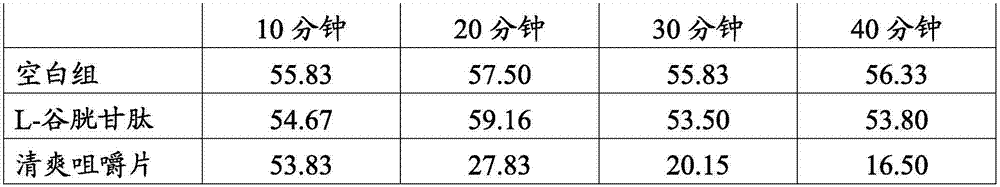
Methods for diagnosing alzheimer's disease
The present invention relates to methods of diagnosing, monitoring, and assessing treatment effects for Aβ amyloidosis, early in the course of clinical disease or prior to the onset of brain damage and clinical symptoms. Methods of measuring the in vivo metabolism of biomolecules produced in the CNS in a subject are provided.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Nano water purifier
InactiveCN1709802ASimple structureCompact structureWater/sewage treatmentEnergy based wastewater treatmentHigh energyGranularity
The invention discloses one kind of nano water clarifier. The nanometer water clarifier includes the outer covering, the lap and the filter element, the filter element supposes for tubularly, the filter element upper extreme supposes for the hemispheroid, the filter element installs in the outer covering, the filter element internal installation has certain energy ceramics pellets, the filter element lower extremity and between the lap hole junction plane where equipped with the seal. Because the nanometer water clarifier filter element and the energy ceramics pellet which includes the nanometer granularity tourmaline, it makes the water through the water clarifier negative ionization and assumes the weak basicity, when the nanometer level granularity tourmaline contacts water, instantaneous causes the water negative ionization. At the same time, activates the water which richly contains the high energy small molecular grouping water, enhanced the water penetrability, the dissolving power, strengthening the human body in vivo metabolism ability. The nanometer purifier also enhanced the water hardness index, which make the water rich in the human body beneficial mineral substance and the trace element.
Owner:耿建平
Sustained/controlled release microsphere of biological extract Genipin cross-linked chitosan coated stilbene compound and preparation method thereof
InactiveCN102240268ALow toxicityUniform qualityAntimycoticsHydroxy compound active ingredientsCross-linkMicrosphere
The invention discloses a sustained / controlled release microsphere of a chitosan coated stilbene compound using a biological extract Genipin as a cross-linking agent and a preparation method thereof. In the microsphere, a cross-linked condensation compound layer of the chitosan and the Genipin serves as a capsule shell and is used for coating a medicament 3,5-dihydroxyl-4-isopropyl stilbene, resveratrol, pterostilbene, oxidized resveratrol or piceatannol; the medicament stability of the product is improved; the sustained / controlled release of the medicament is realized; and the released Genipin after in vivo metabolism of the capsule shell has the characteristics of biocompatibility, no cytotoxicity and the like. The invention is applicable to the sustained / controlled release microspheresof Genipin cross-linked chitosan coated 3,5-dihydroxyl-4-isopropyl stilbene, resveratrol, pterostilbene, oxidized resveratrol, piceatannol and other stilbene compounds and a preparation method thereof. The preparation method is low in requirements on the control condition and easy to operate. In the microspheres, the quality is uniform; the grain size is 0.1 to 100 mu m; the surface of the microsphere has a multiporous structure; and the entrapment rate of the capsule shell to the medicament is 30 to 90 percent.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
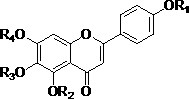
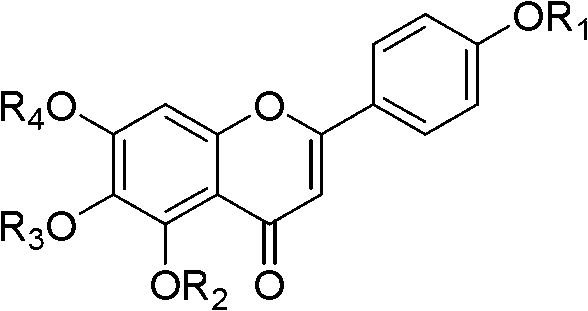
Scutellarin aglycone methylate product based on in-vivo metabolic mechanism as well as preparation method and application of scutellarin aglycone methylate product
InactiveCN102702155AImprove solubilityImprove bioavailabilityOrganic active ingredientsOrganic chemistrySolubilityProduct base
The invention relates to the field of pharmaceutical chemistry study, in particular to a novel scutellarin aglycone methylate product based on in-vivo metabolic mechanism as well as a preparation method of the novel scutellarin aglycone methylate product and an application of the novel scutellarin aglycone methylate product to thrombus prevention and treatment medicine. Pharmacological experiment results show that the scutellarin aglycone methylate product provided by the invention has pharmacological effects of better solubility, antioxidation, cell damage inhabitation and the like, and can be developed into novel medicine for preventing and treating thrombotic diseases.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
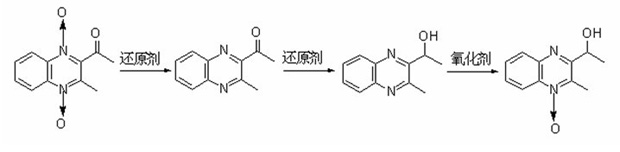
1-oxo-2-methyl-3-(1-ethoxyl)-quinoxaline and preparation method and application thereof
InactiveCN102070539ARaw materials are cheap and easy to getMild reaction conditionsOrganic chemistryComponent separationBiotechnologyQuinoxaline
The invention discloses 1-oxo-2-methyl-3-(1-ethoxyl)-quinoxaline, a preparation method and application thereof. The 1-oxo-2-methyl-3-(1-ethoxyl)-quinoxaline has a structure shown as a formula (I). The method for preparing the product (I) comprises the steps of: taking maquindox as a raw material, and obtaining 3-methyl-2-(acetyl)-quinoxaline through a reduction reaction; then obtaining 2-(1-ethoxyl)-3-methyl-quinoxaline through a one-step reduction reaction; and finally, reacting an obtained product with an oxidant to obtain the product (I). The product (I) can be taken as a residual marker of the metabolism of the maquindox in the bodies of target animals or a standard substance or reference substance for detecting maquindox residue in animal derived food.
Owner:GENIFARM LAB INC +1
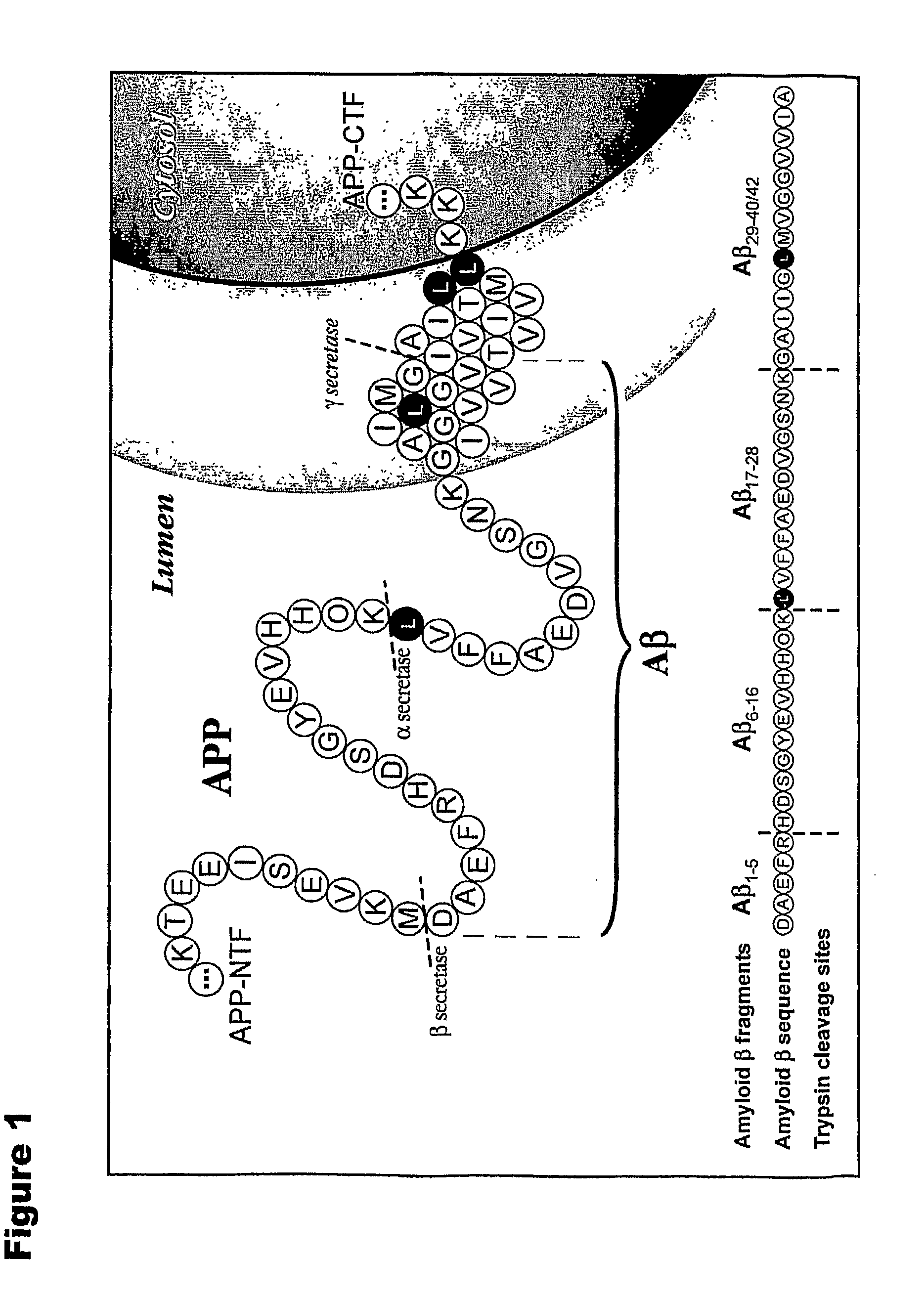
Methods for measuring the metabolism of neurally dervied biomolecules in vivo
The present invention relates to methods of diagnosing, monitoring, and assessing treatment effects for neurological and neurodegenerative diseases and disorders, such as Alzheimer's Disease, early in the course of clinical disease or prior to the onset of brain damage and clinical symptoms. Methods of measuring the in vivo metabolism of biomolecules produced in the CNS in a subject are provided.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Wellness enzyme beverage capable of promoting in-vivo metabolism and coordinating intestine and stomach functions and preparation method thereof
InactiveCN103300447APromote peristalsisImprove sleepingFood preparationIntestinal structureAdditive ingredient
The invention relates to a wellness enzyme beverage and a preparation method thereof, and in particular relates to a wellness enzyme beverage capable of promoting in-vivo metabolism and coordinating intestine and stomach functions and a preparation method thereof, belonging to the technical field of nutritional and health-care foods. The beverage is characterized in that each 1000 parts of the wellness enzyme preparation comprises the following components in parts by mass: 200-350 parts of comprehensive plant enzyme stock solution, 5-15 parts of fructo-oligosaccharide pulp, 1-5 parts of bifidobacterium flora, 1-5 parts of vitamin B complex, 10-15 parts of corn starch, 5-10 parts of maltodextrin and the balance of purified water. The beverage disclosed by the invention has the advantages that the wellness enzyme components are reasonable in proportion, the synergistic effect of the components is remarkable, the in-vivo metabolism can be obviously promoted and the intestine and stomach functions can be effectively coordinated; and the production process is simple and easy to operate, so that the beverage is suitable for industrial production and has a good economic benefit and social benefit.
Owner:湖北汇丰医药科技有限公司
Preparation method of sugar-free moon-cake syrup
InactiveCN102823801ASimple processMild production conditionsDough treatmentFood preparationFood additiveAdditive ingredient
The invention discloses a preparation method of sugar-free moon-cake syrup, and belongs to the field of food additives. The sugar-free moon-cake syrup is prepared from maltitol, xylitol and erythritol serving as raw materials by a specific process, and the process for producing the product does not require boiling sugar, so that labor and time are saved; and saccharification does not exist in in-vivo metabolism and is independent of insulin, so that the problems that a patient with diabetes mellitus cannot taste sweet foods can be solved, and glucose and insulin in blood are prevented from greatly fluctuating. The moon-cake produced by applying the syrup has smooth and fine surface, high nutrition and gold greasy color. The method for using the sugar-free moon-cake syrup comprises the step of doping with flour in a sugar-free moon-cake syrup-flour ratio of 4:5 to produce the Cantonese moon-cake crust when a Cantonese moon-cake is made.
Owner:JIANGSU HENGHUI FOOD CO LTD
Oil-in-water type spearmint oil nano emulsion and method for preparing same
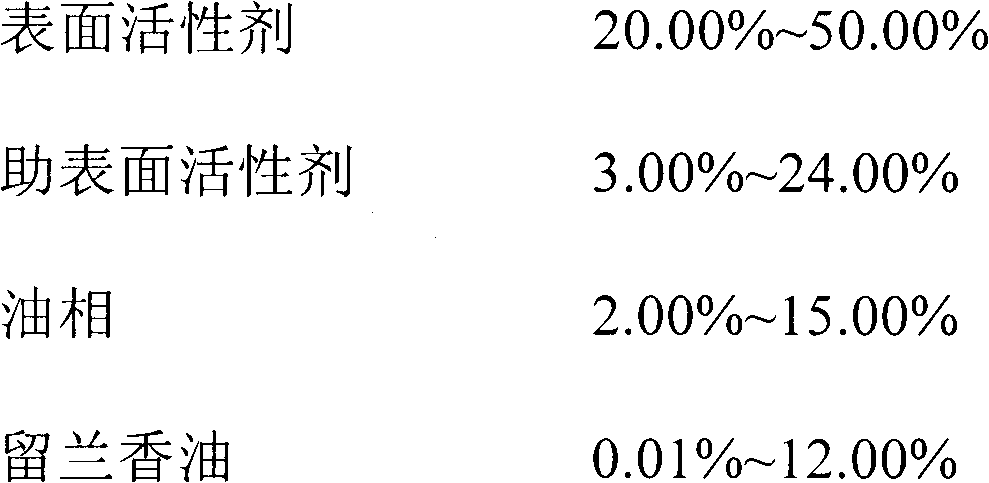
InactiveCN102166254AHigh thermodynamic stabilityEasy to operateNervous disorderDigestive systemIn vivo metabolismSolubility
The invention discloses oil-in-water type spearmint oil nano emulsion. The nano emulsion is 1 to 100nm in grain size and consists of the following raw materials in percentage by weight: 20.00 to 50.00 percent of surfactant, 3.00 to 24.00 percent of cosurfactant, 2.00 to 15.00 percent of oil phase, 0.01 to 12.00 percent of spearmint oil and the balance of distilled water. The nano emulsion is yellow or colorless, clarified and transparent liquid, has high stability, and can improve the medical stability of spearmint oil during processing, improve the solubility and the bioavailability of spearmint oil, prolong the in vivo metabolism time of spearmint oil and enjoy a wide market prospect in medical field.
Owner:NORTHWEST A & F UNIV
7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and preparation method thereof
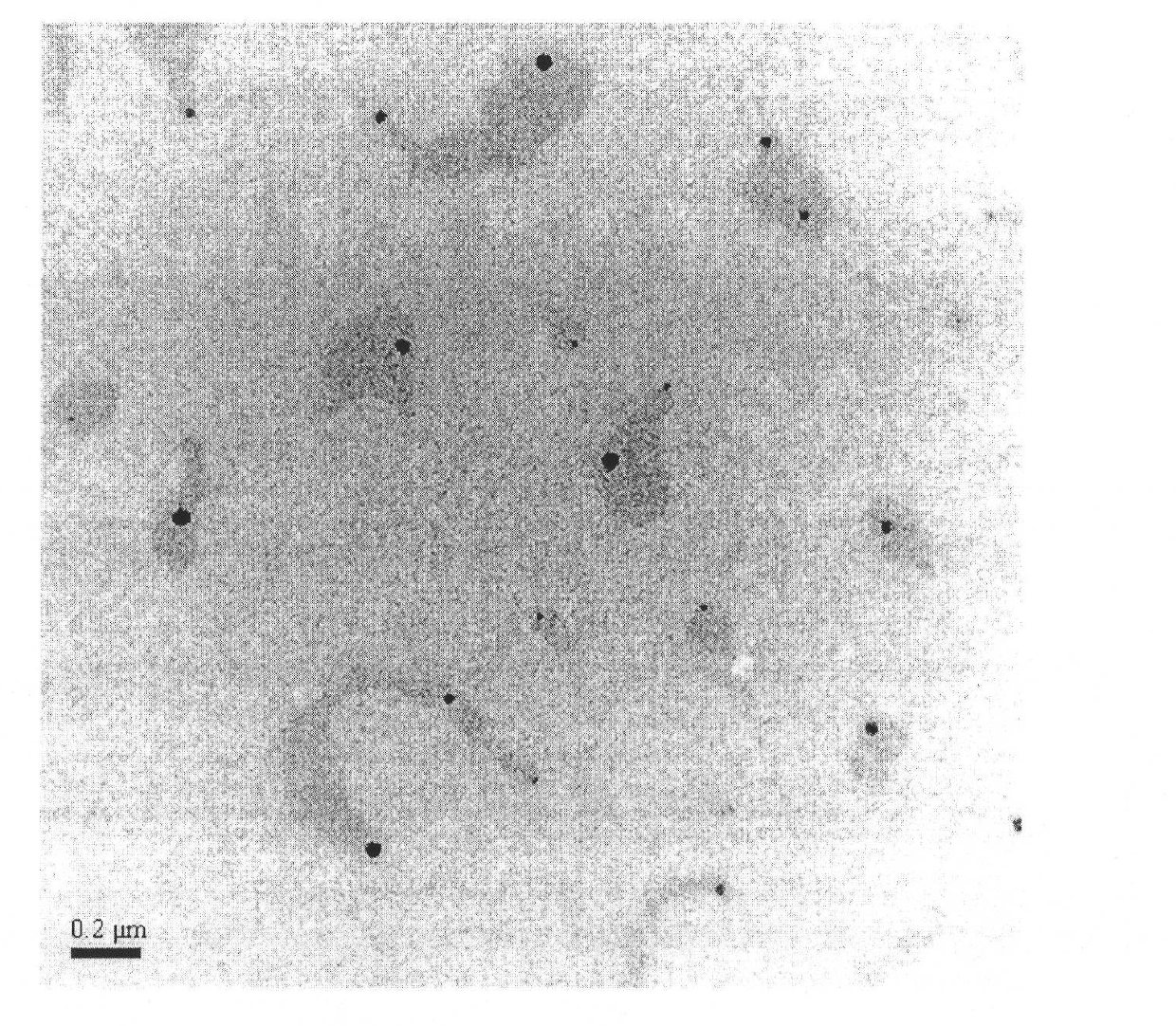
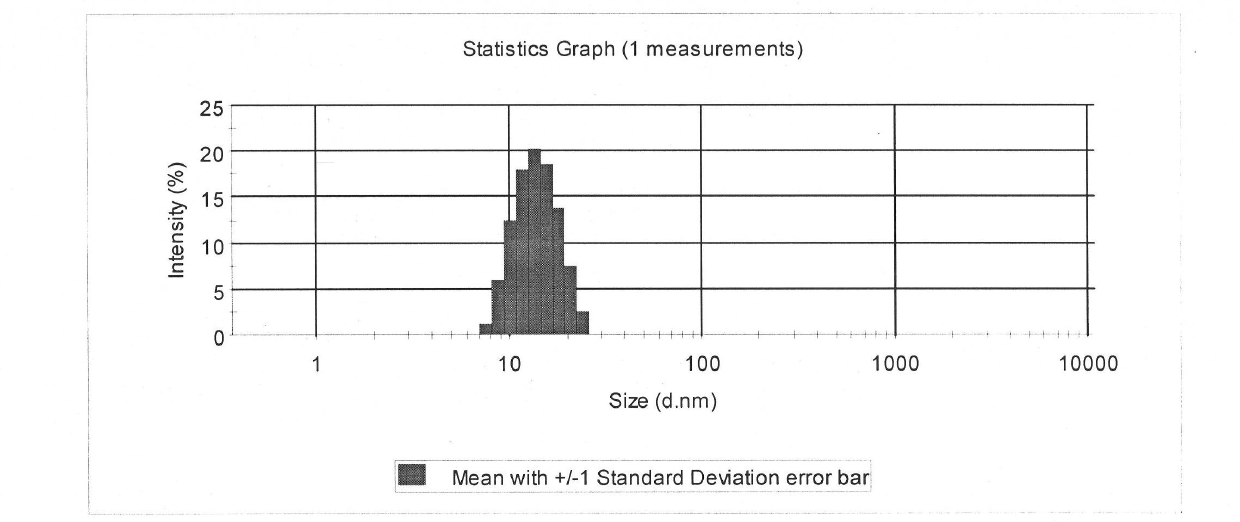
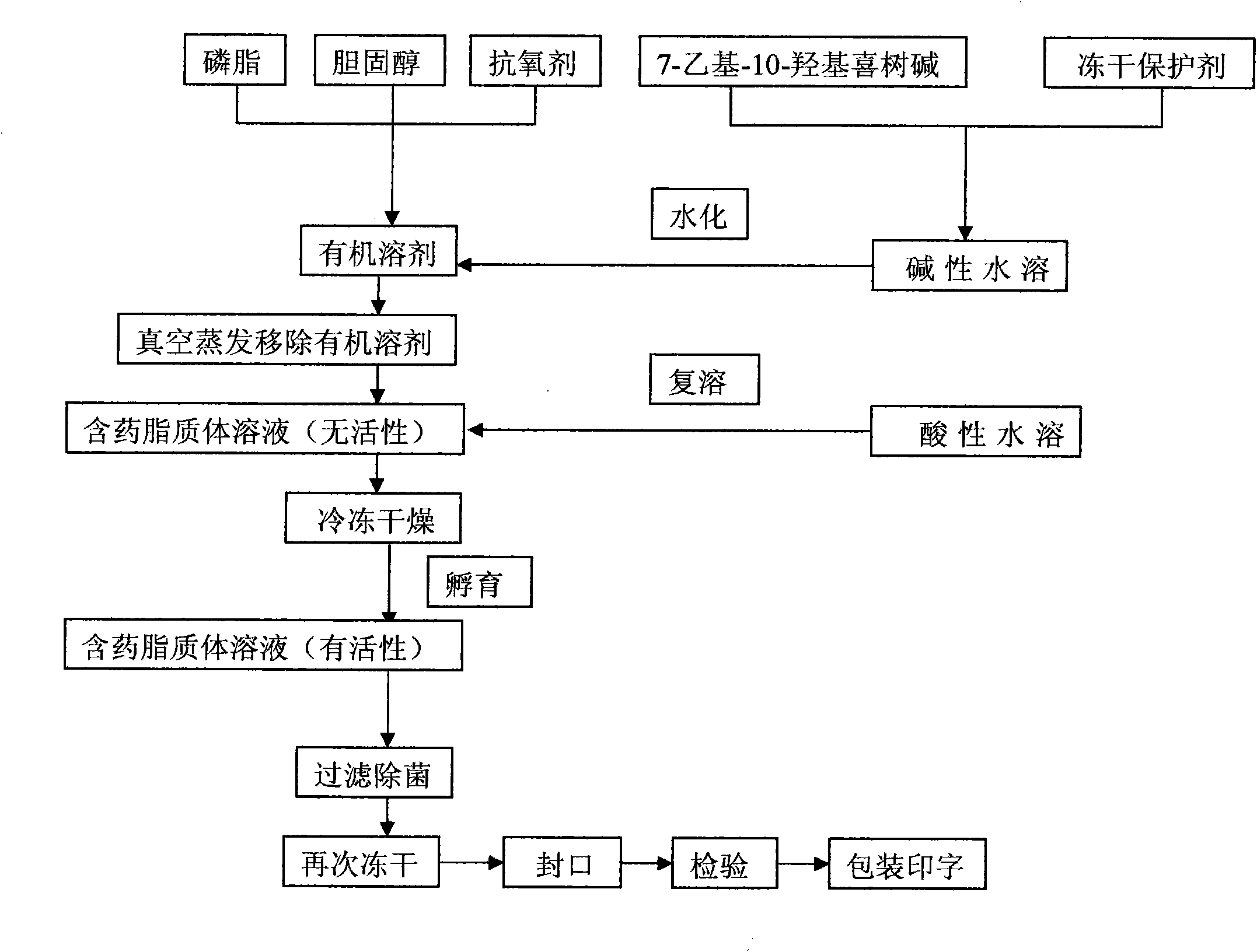
InactiveCN101874788AImprove stabilityImprove in vivo stabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the medical technical field, and discloses 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and a preparation method thereof. The 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection comprises the following components: 1-10g of 7-ethyl-10-hydroxycamptothecine, 30-60g of phospholipids, 10-40g of cholesterol, 2-8g of VE, 100-300g of a freeze drying protectant, 2000-8000ml of an organic solvent, 1000-4000ml of alkaline buffer salt solution and 1000-4000ml of acid buffer salt solution. The preparation method comprises the following steps: dissolving liposoluble components in the organic solvent and water-soluble components in the alkaline buffer salt; transferring the organic solvent, and then adding the alkaline buffer salt for hydration; and carrying freeze drying in vacuum, re-dissolving with the acid buffer salt, incubating, filtering, sterilizing, and carrying out freeze drying again to obtain the 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection for injection. The invention solves the problems of low solubility and fast in-vivo metabolism of the 7-ethyl-10-hydroxycamptothecine, thus lowering toxic reaction, eliminating side reaction, having higher target distribution characteristics, prolonging metabolism time and improving solubility and bioavailability.
Owner:SHENYANG PHARMA UNIVERSITY
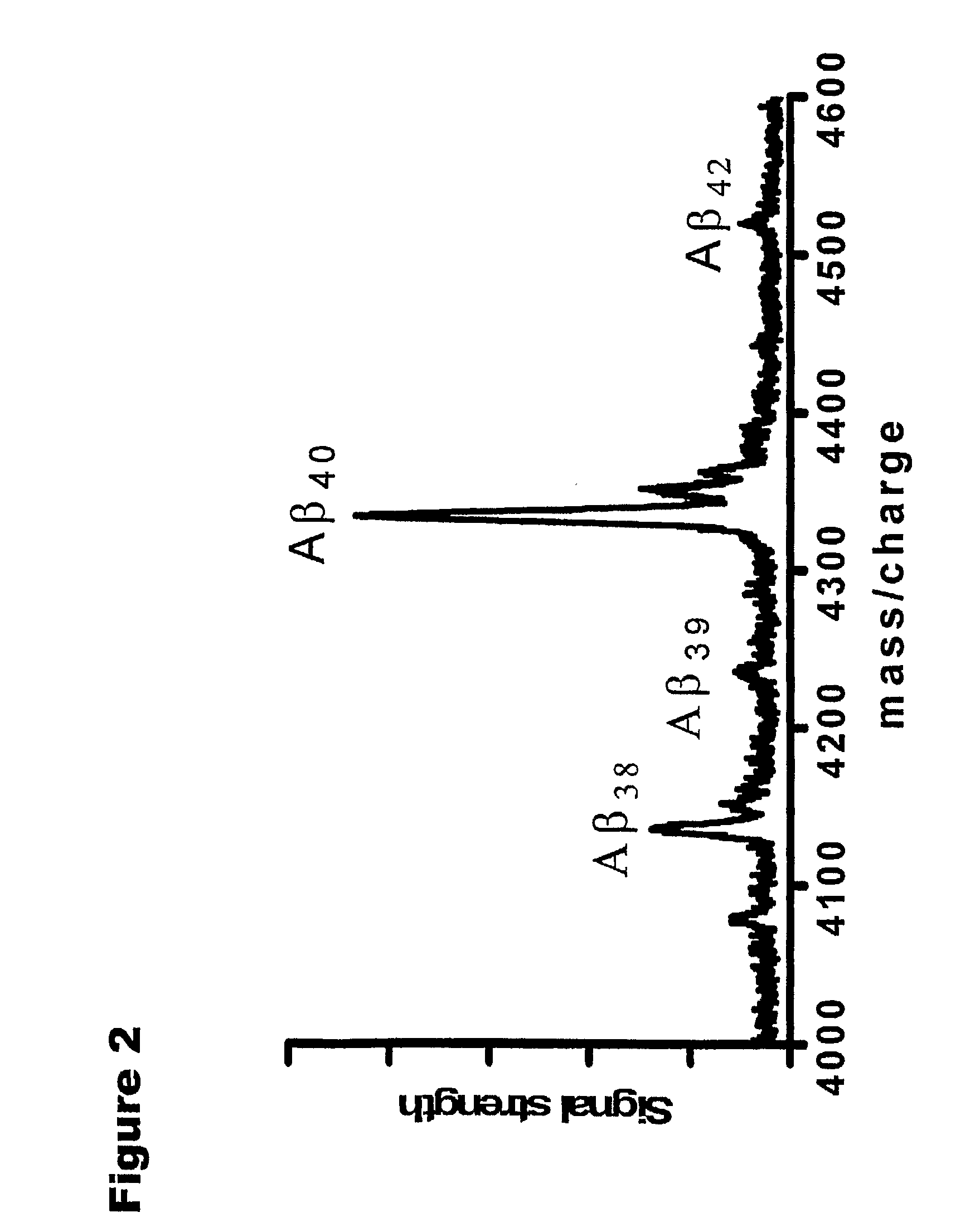
Methods for Measuring Concentrations of Biomolecules
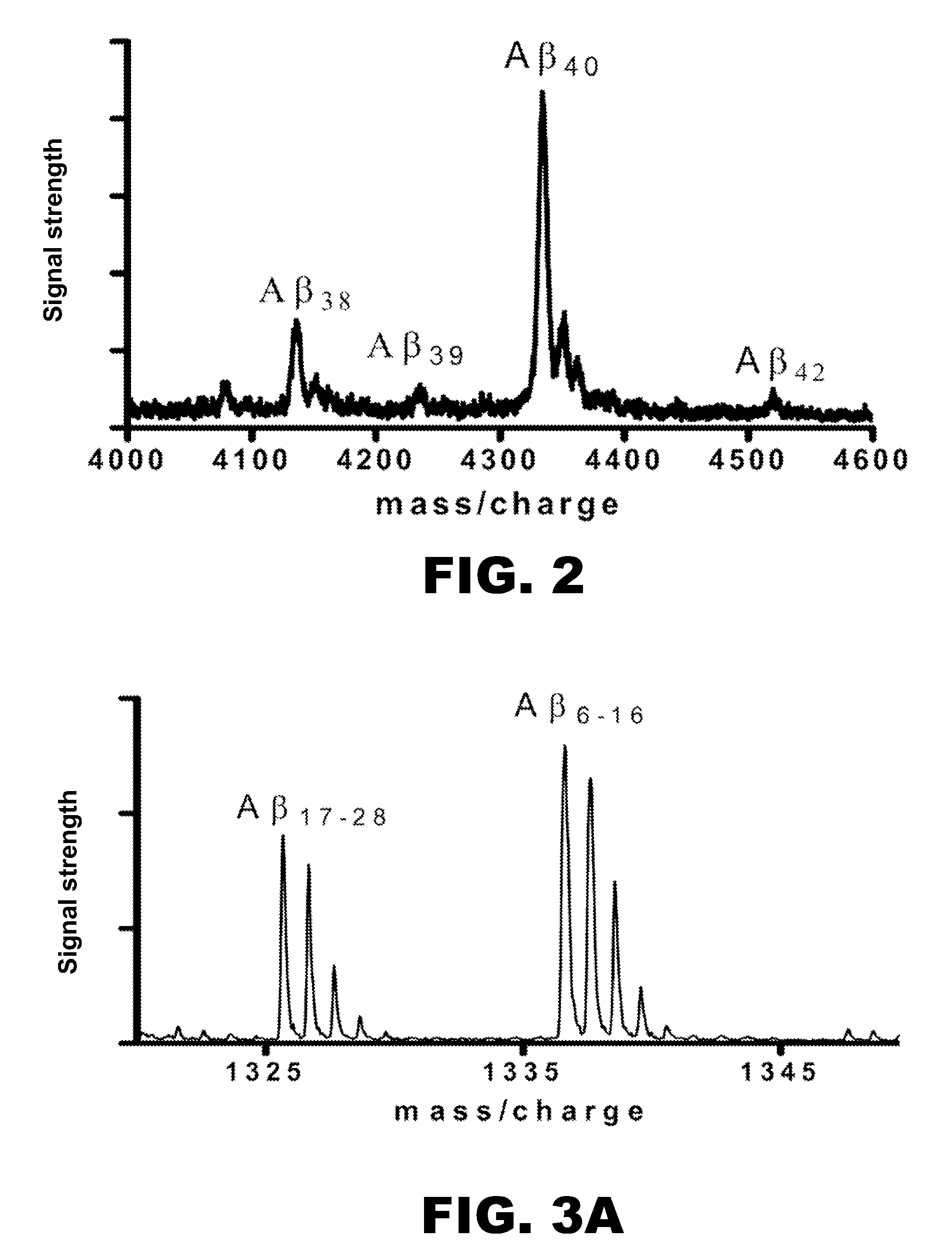
The present invention provides methods for measuring the absolute concentration of a biomolecule of interest in a subject. Such biomolecules may be implicated in one or more neurological and neurodegenerative diseases or disorders. Also provided is a method for determining whether a therapeutic agent affects the in vivo metabolism of a central nervous system derived biomolecule. Also provided are kits for performing the methods of the invention.
Owner:C2N DIAGNOSTICS
A kind of prepared wine and its preparation method and application
ActiveCN102258718AMeet \"junMeet the minister, assistantSenses disorderNervous disorderDiseaseIron-deficiency anemia
The invention discloses an integrated alcoholic beverage, which comprises base liquor and additives, and is characterized by comprising 70-80 percent of base liquor and 20-30 percent of additives, wherein the base liquor is distilled liquor; the additive comprise extracts of the following medicinal materials in parts by weight: 20-50 parts of platycodon root, 20-60 parts of cinnamon, 15-50 parts of lily, 15-80 parts of medlar, 10-80 parts of lotus seed, 20-100 parts of hawthorn, 20-80 parts of coix seed and 10-50 parts of malt. The additives have various effects of nourishing, treatment and the like and meet the principle of monarch, minister, assistant and guide of Chinese medicine, so that the integrated alcoholic beverage has complete functions and quick response. A product provided by the invention has the effects of enhancing resistance, invigorating the circulation of blood, enriching the blood, adjusting a human immunologic mechanism, correcting in-vivo metabolism, eliminating fatigue, softening blood vessel and resisting aging and can be used as an auxiliary therapeutic agent of some tumors, senile diseases, urinary diseases, gynecological diseases, traumatic injury and iron deficiency anemia.
Owner:贵州长寿长乐原生食品有限公司
Gelatin-blood plasma and its preparing method
InactiveCN1457881AIncrease or restore blood volumeNormal proteinHydrolysed protein ingredientsUnknown materialsSodium lactateSide effect
The present invention relates to a kind of gelatin as blood plasma substitute and its preparation process. The blood plasma substitute is prepared with gelatin extracted from fur seal skin as material and possessing molecular weight 15000-36000. The blood plasma substitute consists of pure fur seal gelatin 29-31 g, sodium chloride for injection 5.37-5.39 g, potassium chloride 0.36-0.38 g, magnesium chloride 0.13-0.15 g, sodium lactate 3.35-3.37 g and distilled water 1000 ml. The blood plasma substitute is made to participate metabolism in body; has the functions of expanding blood capacity, maintaining essential osmotic pressure and improving blood circulation; and may be injected together with other intravenous injection to produce obvious curative effect and no toxic side effect.
Owner:刘威
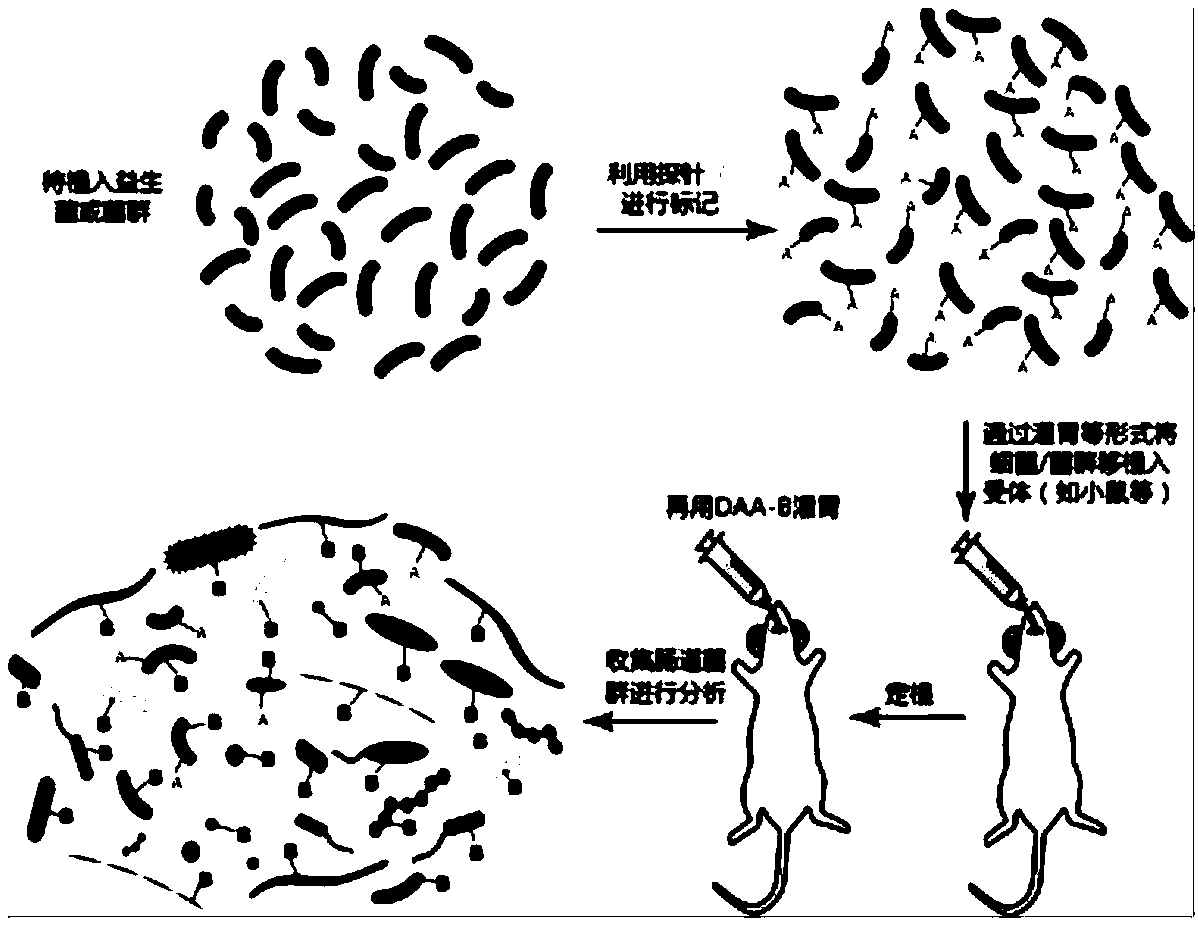
Method for evaluating activity of bacteria after transplanting bacteria into digestive tract
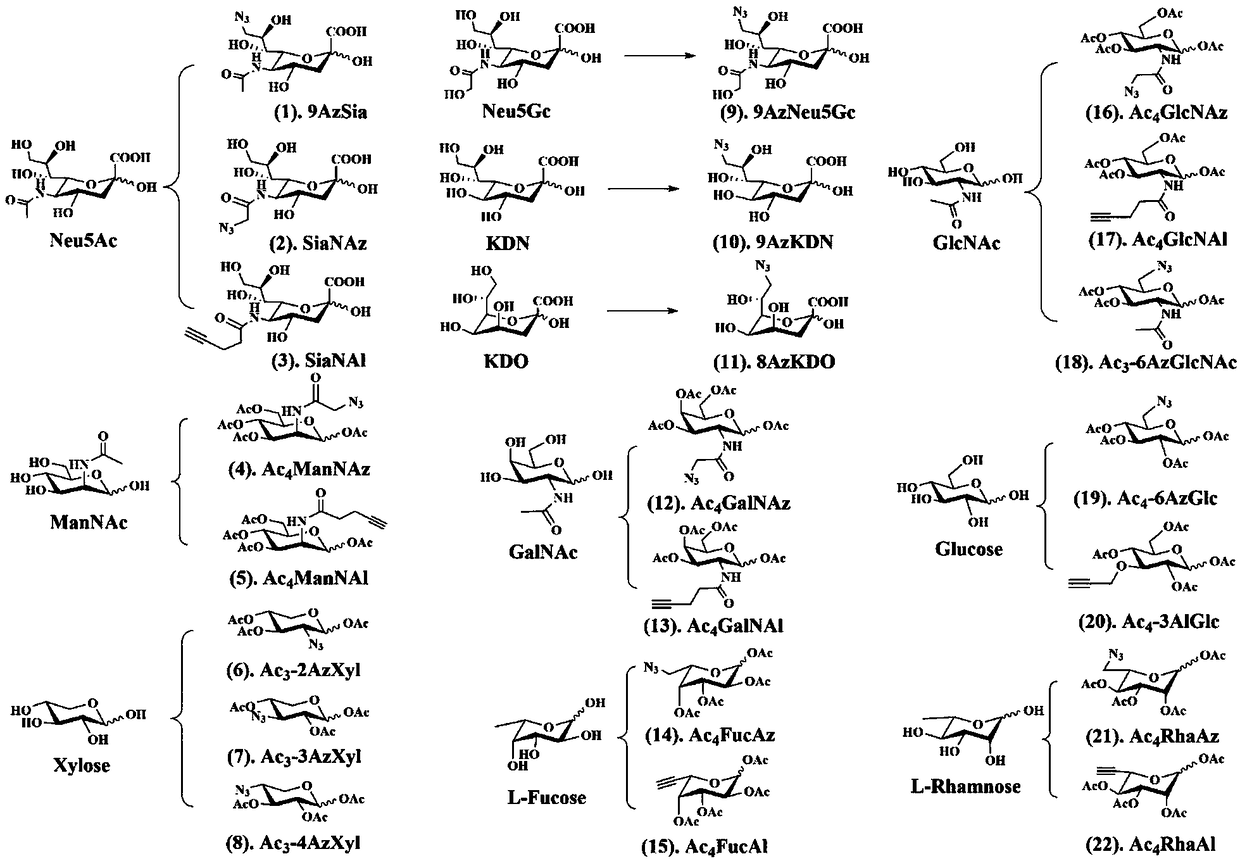
The invention relates to a method for evaluating the activity of bacteria after transplanting the bacteria into a digestive tract. The method comprises the following steps: enabling implantation bacteria before transplanting to carry A-group markers; marking and treating an intestinal flora of a receptor receiving the implantation bacteria by utilizing in-vivo metabolism of B-group markers after transplanting the implantation bacteria into the digestive tract for a certain time, thus detecting bacteria which simultaneously carry the A-group markers and the B-group markers and bacteria which just carry one of the A-group markers and the B-group markers of the flora.
Owner:RENJI HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
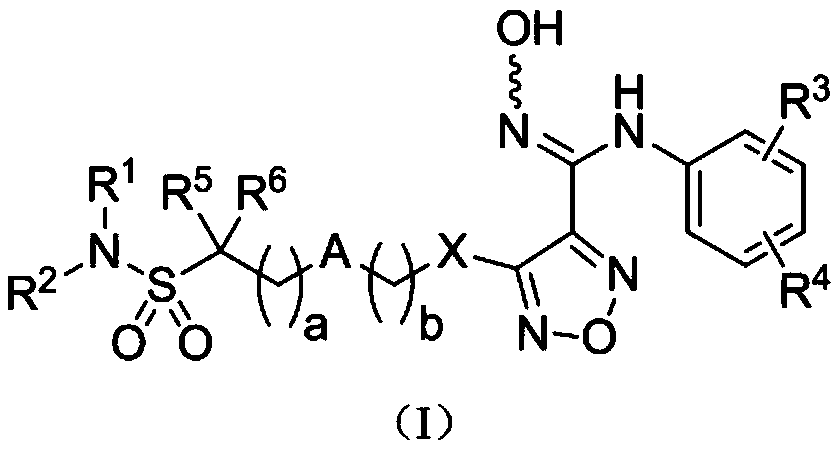
Indoleamine-2,3-dioxygenase inhibitor, preparation method and uses thereof
PendingCN110128367AEnhanced inhibitory effectImprove pharmacokineticsOrganic active ingredientsSenses disorderDiseaseTryptophan
The present invention provides a compound represented by a formula (I), and relates to a pharmaceutical composition containing the compound represented by the formula (I), and uses of the compound inpreparation of indoleamine-2,3-dioxygenase (IDO) inhibitor drugs. According to the present invention, the prepared compound has obvious inhibitory effect on IDO protease, and is metabolically stable in vivo; and the compound or the pharmaceutical composition thereof can be used for the preparation of IDO inhibitor drugs, and can further be used for the preparation of drugs for preventing and / or treating diseases having pathological characteristics of IDO-mediated tryptophan metabolism pathway.
Owner:HINOVA PHARM INC
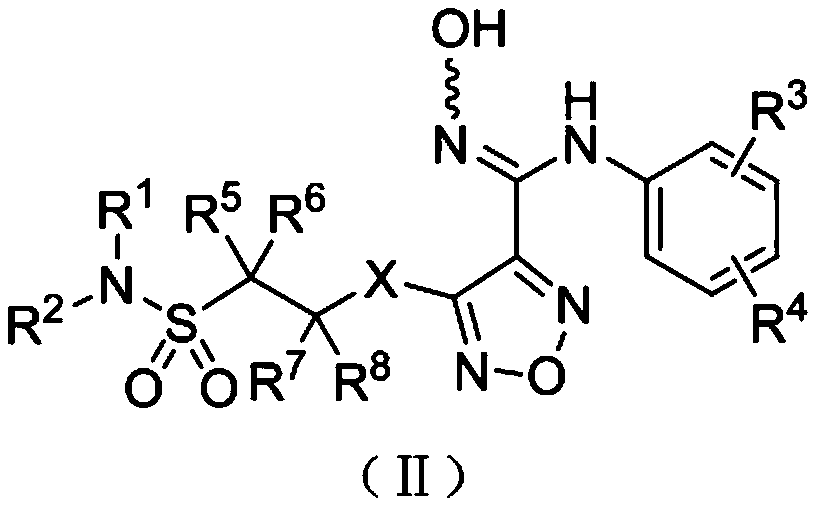
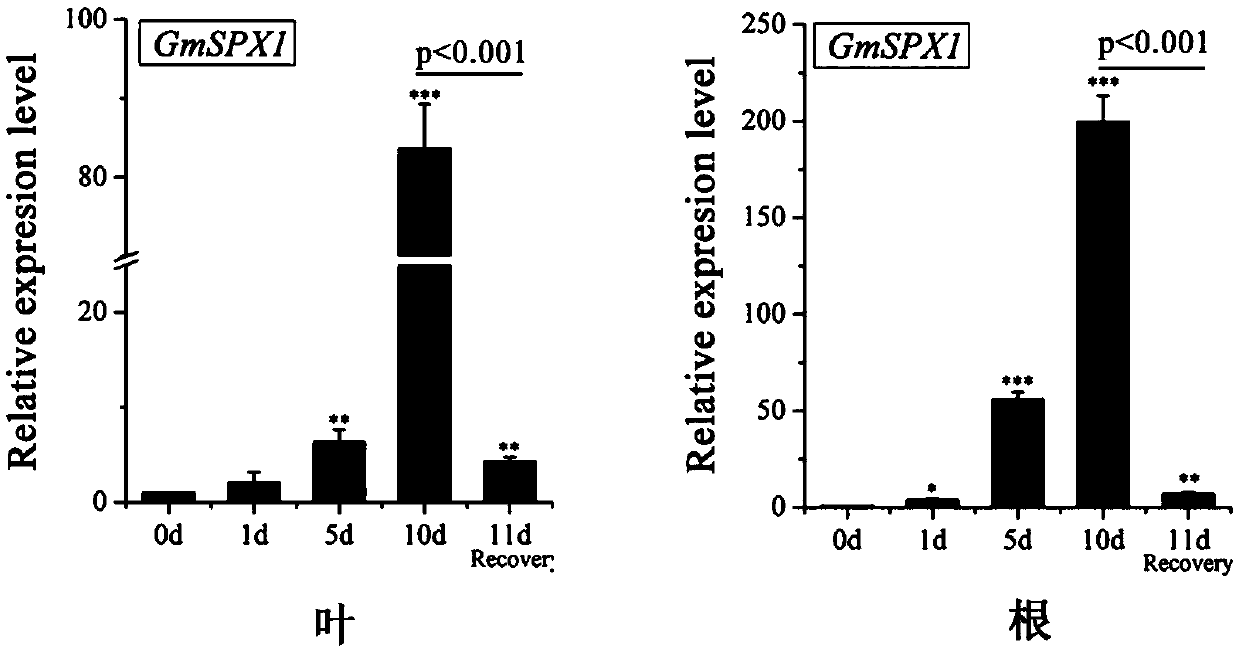
Soybean phosphate-starvation negative regulatory gene GmSPX1, and coding protein and application thereof
InactiveCN105505948APositive regulationTotal Phosphorus ReductionPlant peptidesFermentationPhosphateNucleotide
The invention belongs to the technical field of gene engineering, and particularly discloses a phosphate-starvation negative regulatory gene GmSPX1, and a coding protein and application thereof. The nucleotide sequence of the gene is disclosed as Seq ID NO.1. The invention detects that any plant expression vector capable of inserting the exogenous gene GmSPX1 can be used for transforming plant cells to obtain the GmSPX1-overexpressed transgenic plant. Compared with the non-transgenic plant, the complete phosphorus content of the transgenic plant is obviously reduced, the phosphate-starvation related gene is obviously lowered, the root system morphology is obviously changed, and the phosphorus poisoning phenomenon of the mutant is relieved. The gene disclosed by the invention can be inserted into the plant as a target gene, enhances the phosphorus in-vivo metabolic balance capacity of the transgenic plant, and thus, has important meanings for culturing phosphorus-efficient-utilization soybean varieties.
Owner:NANJING AGRICULTURAL UNIVERSITY
Influenza virus replication inhibitor and uses thereof
ActiveCN111057074AEnhanced inhibitory effectLow cytotoxicityOrganic chemistryAntiviralsIn vivo metabolismPharmaceutical medicine
The invention belongs to the field of medicines, and particularly relates to a new compound serving as an influenza virus replication inhibitor, a preparation method thereof, a pharmaceutical composition containing the compound, and application of the compound and the pharmaceutical composition in the treatment of influenza. The compound is a compound shown in a formula (I) or a stereoisomer, a tautomer, oxynitride, a solvate, a metabolite, a pharmaceutically acceptable salt or prodrugs of the compound shown in the formula (I). The compound can well inhibit influenza viruses, and / or has lowercytotoxicity, more excellent in-vivo metabolic dynamics properties and in-vivo pharmacodynamic properties.
Owner:SUNSHINE LAKE PHARM CO LTD
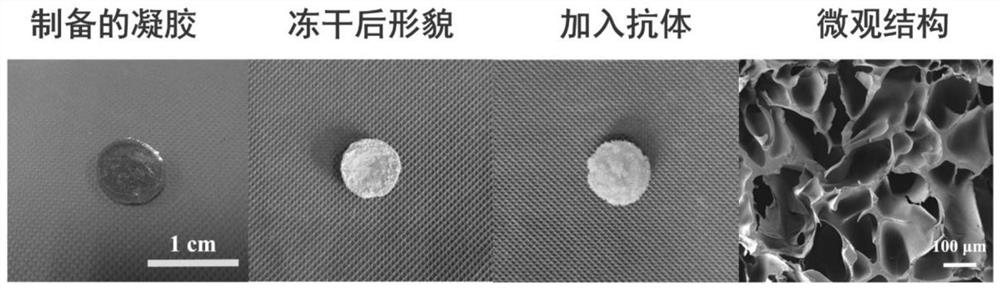
Scaffold material as well as preparation method and application thereof
ActiveCN111803454ASimple and gentle preparationIncrease profitPowder deliveryOrganic active ingredientsPolyethylene glycolBiocompatibility
The invention provides a scaffold material. The scaffold material is obtained by cross-linking multi-arm amino polyethylene glycol and multi-aldehyde glucan. Compared with the prior art, according tothe scaffold material provided by the invention, a cross-linked grid is formed through the Schiff base effect of aldehyde groups and amino groups; and when a medicine is carried, the medicine can be packaged in a scaffold, and the aldehyde groups rich in the material can be coupled with the amino-containing medicine. Therefore, the medicine is effectively retained in the cross-linked grid; furthermore, the medicine can be locally and slowly released at the focus part, and the problems of quick in-vivo metabolism and low utilization rate of the medicine are effectively solved. The scaffold material has wide development prospects in the application aspects of maintaining in-vivo drug concentration, reducing administration dosage, improving drug utilization rate, avoiding drug resistance andthe like. The scaffold material is simple and mild in preparation, free of selectivity for supported drug types, wide in application range and good in biocompatibility.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
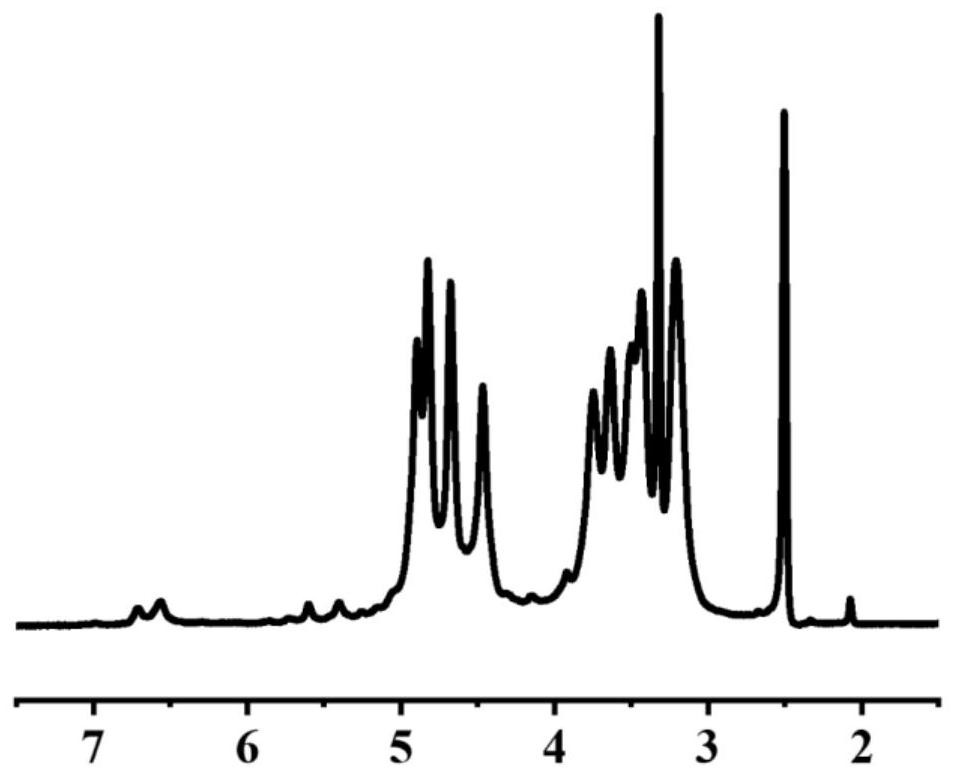
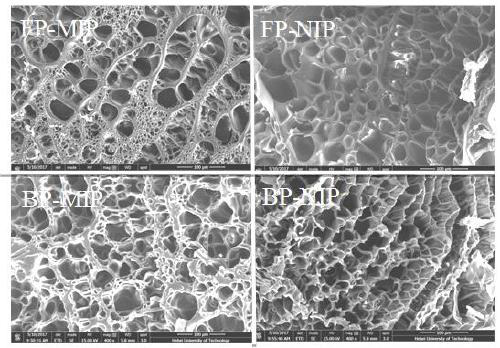
Frontal polymerization preparation method for thymopentin molecular imprinted hydrogel
The invention relates to a frontal polymerization preparation method for a thymopentin molecular imprinted hydrogel which is prepared from the raw materials in percentage by mass: 4.33%-11.94% of thymopentin, 10.00%-36.44% of N-isopropylacrylamide, 5.88%-21.86% of acrylic acid, 0.36%-0.74% of N, N'-methylenebisacrylamide, 9.92%-33.58% of choline chloride and 30.12%-53.68% of ethylene glycol. By using the frontal polymerization preparation method, a molecular imprinted hydrogel sustained-release material is prepared by taking bioactive molecule thymopentin as a template and a green solvent DESsystem as a pore-foaming agent, and the molecular imprinted hydrogel sustained-release material serving as a drug carrier is stable in physical and chemical properties and obvious in imprinting effect; the molecular imprinted hydrogel sustained-release material is large in drug loading capacity and has an obvious sustained-release effect for the template molecule thymopentin; and compared with a thermal polymerization imprinted hydrogel, the molecular imprinted hydrogel sustained-release material is capable of prolonging the in-vivo metabolism time of a drug and increasing the biological availability of the drug.
Owner:TIANJIN MEDICAL UNIV
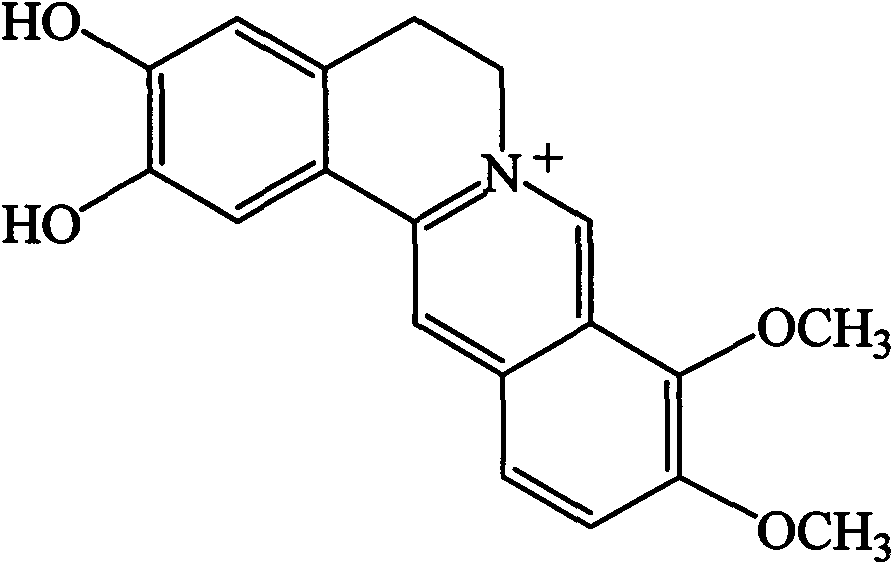
Application of demethyleneberberine in preparation of hypolipidemic drug
InactiveCN103919774AFat-lowering effect is goodOrganic active ingredientsMetabolism disorderSide effectMetabolite
The invention provides a hypolipidemic compound demethyleneberberine. The demethyleneberberine is derived from a main in-vivo metabolite of natural medicine berberine. The researches on model animals with hyperlipidemia find that the demethyleneberberine has good drug effects of reducing cholesterol, triglyceride, low density lipoprotein (LDL) and the like, and has no obvious side effects for 6 weeks of continuous medication. The discovery can lead to discovery of a new hypolipidemic drug, and provides another alternative medicine for clinic treatment of hyperlipidemia, lipid metabolism disorder and high fat and obese patients and related people wanting to lose weight.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com