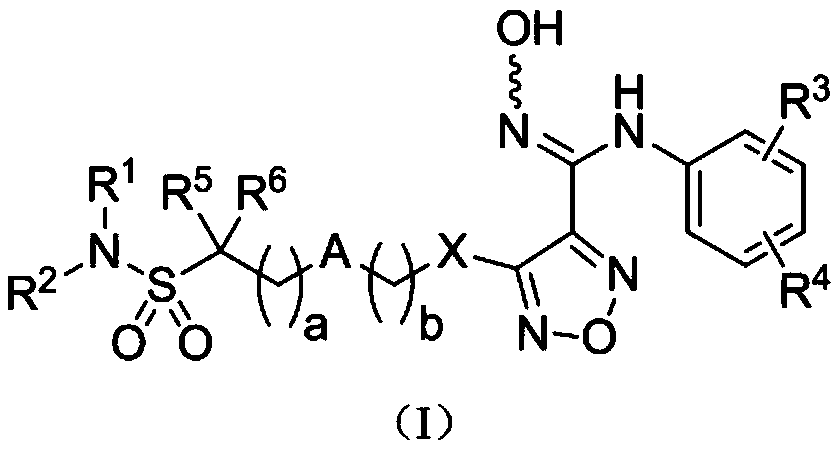
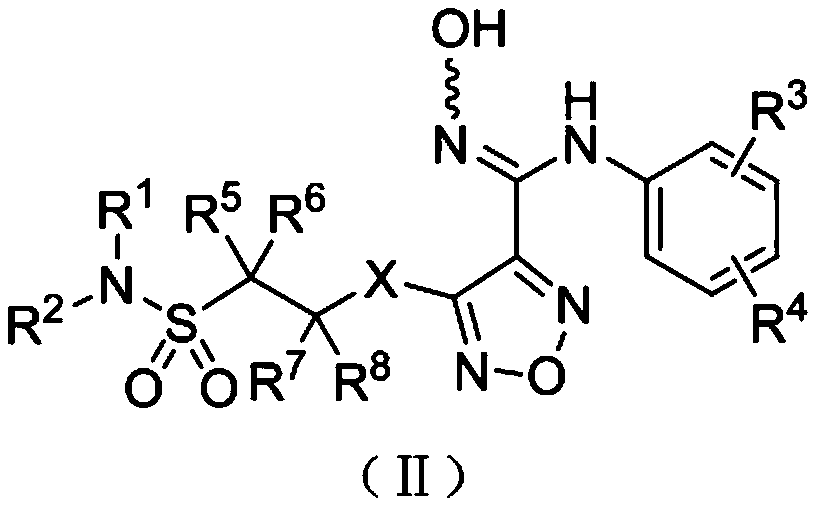
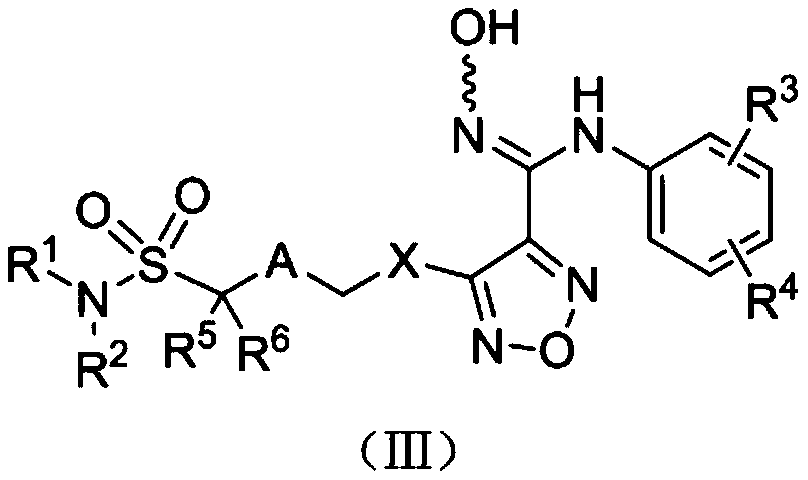
Indoleamine-2,3-dioxygenase inhibitor, preparation method and uses thereof
A C3-C7, solvate technology, applied in the field of medicinal chemistry, can solve the problems of drug metabolism, stability, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((2-sulfamoylethyl)amino)-1,2,5-oxadiazole-3- Methylamine (1)
[0109]
[0110] The first step: synthesis of intermediate 1-1:
[0111] Compound SM1-1 was prepared by literature method (WO 2017106062). The raw material SM1-1 (0.1mmol, 37mg) and 2-aminoethanesulfonamide hydrochloride (0.2mmol, 32mg, Shanghai Bi De Pharmaceutical Co., Ltd.) were dissolved in 2mL of THF, and 0.5mL of saturated sodium bicarbonate solution was added to react at room temperature overnight. The reaction solution was extracted with ethyl acetate, and the organic phase was washed with 0.5N hydrochloric acid and then with saturated brine. After the organic phase was separated, the organic layer was concentrated under reduced pressure. The obtained crude product was purified by thin-layer chromatography to obtain 22 mg of a white solid (Intermediate 1-1), with a yield of 48%.
[0112] The second step: synthesis of compound 1:
[0113] Int...
Embodiment 2
[0116] Example 2: N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((2-(N-methylsulfamoyl)ethyl)amino)-1,2,5- Oxadiazole-3-methimidine (2)
[0117]
[0118] The first step: synthesis of intermediate 2-1:
[0119] Intermediate 1-1 (113mg, 0.25mmol) and potassium carbonate (70mg, 0.5mmol) were sequentially added to DMF (5mL), then methyl iodide (36mg, 0.25mmol) was added slowly, stirred overnight at room temperature, TLC monitored the reaction materials disappear. Add 10 mL of water, extract with 20 mL of ethyl acetate, wash with saturated brine, dry over anhydrous sodium sulfate, purify by prep-TLC, develop with ethyl acetate / n-hexane=1 / 1, and obtain 20 mg of intermediate 2-1, yield: 17%.
[0120] Intermediate 2-1 (20 mg, 0.04 mmol) was added to 5 mL of tetrahydrofuran, 1.5 mL of sodium hydroxide (2N) solution was added, and the reaction was stirred at room temperature for 30 minutes, and the reaction raw materials basically disappeared as monitored by TLC. Add 10 mL of water, ex...
Embodiment 3
[0122] Example 3: N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((2-(N-ethylsulfamoyl)ethyl)amino)-1,2,5- Oxadiazole-3-methimidine (3)
[0123] Compound 3 was prepared by a synthesis method similar to Example 1 using SM1-1 and SM2-3 as raw materials.
[0124]
[0125] Step 1: Synthesis of SM2-3:
[0126] Ethylamine hydrochloride (81.5mg, 1.0mmol) was dissolved in 5mL DCM, triethylamine (303mg, 3.0mmol) was added, and 2-benzenedi(form)imidoethanesulfonyl chloride (273mg , 1.0 mmol), reacted for 15 min, and monitored the reaction by TLC until the disappearance of the starting material. Added 10 mL of water, extracted with 10 mL of DCM, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, and purified by thin layer chromatography (DCM / MeOH=10:1) to obtain 65 mg of a white solid product. Rate: 23%.
[0127] Dissolve the white solid product (65mg, 0.23mmol) in 5mL of ethanol, add hydrazine hydrate (17.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com