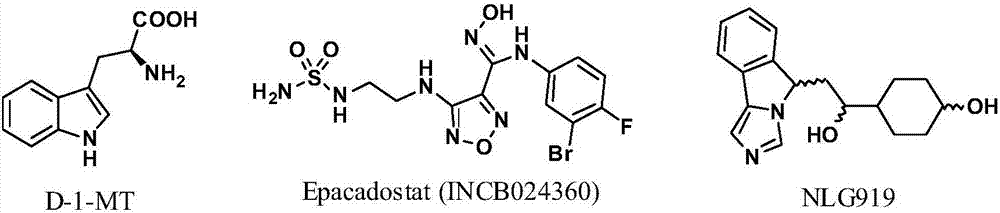
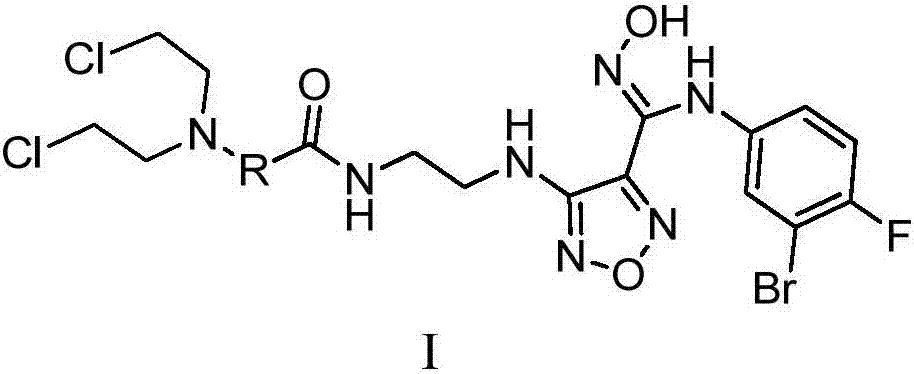
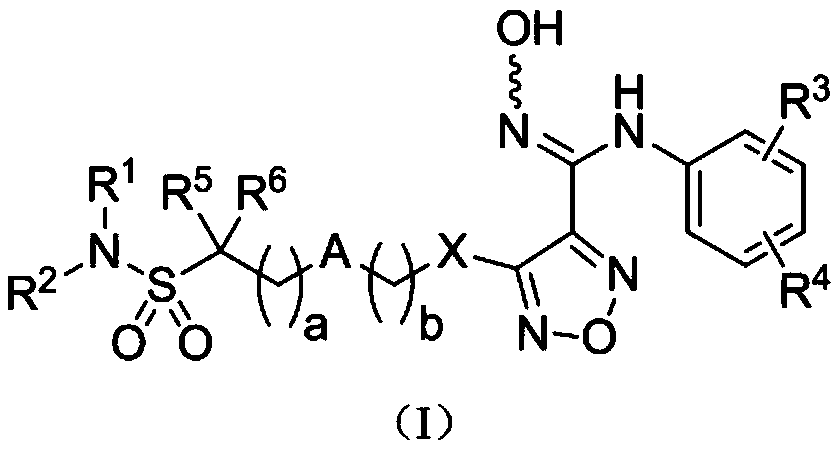
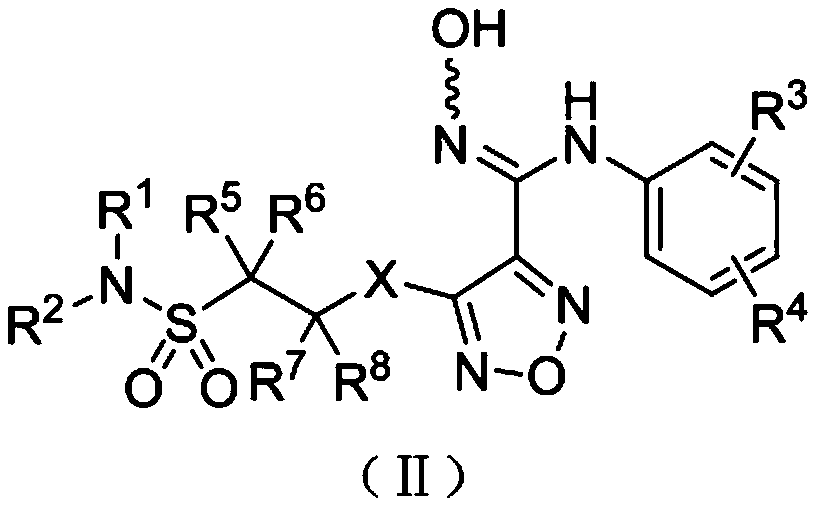
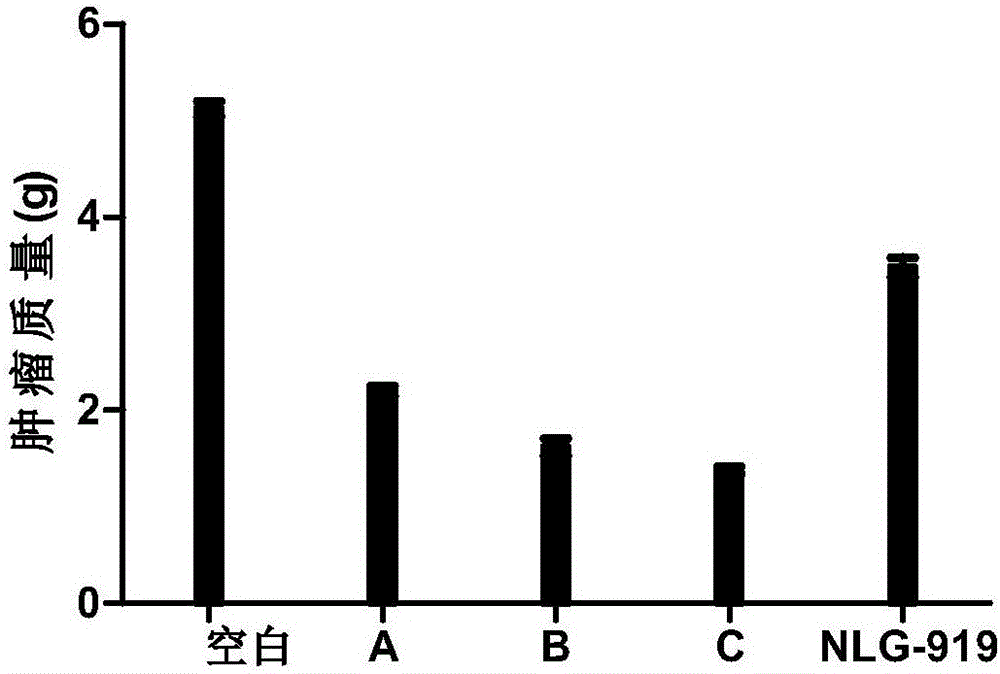
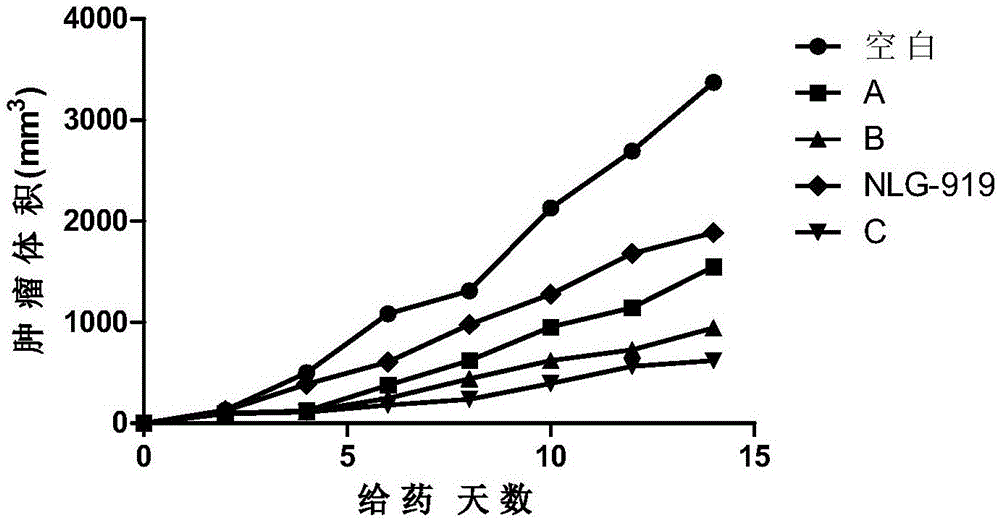
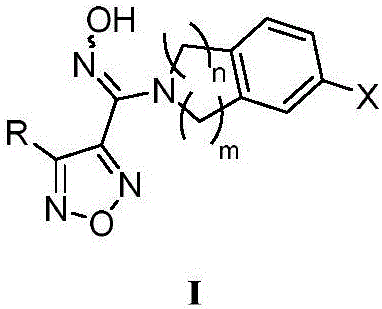
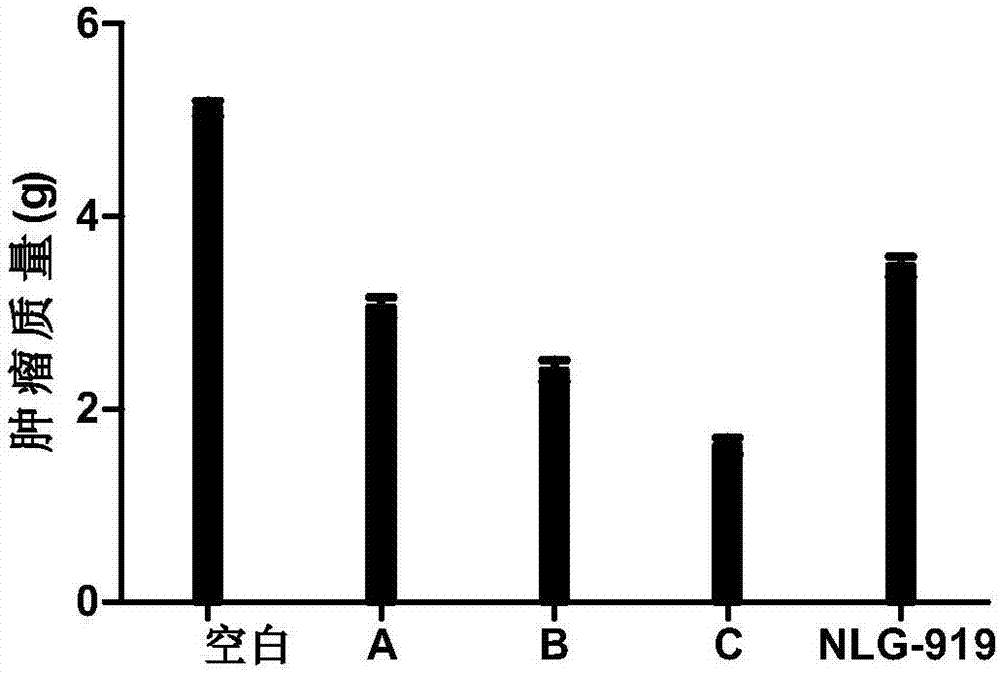
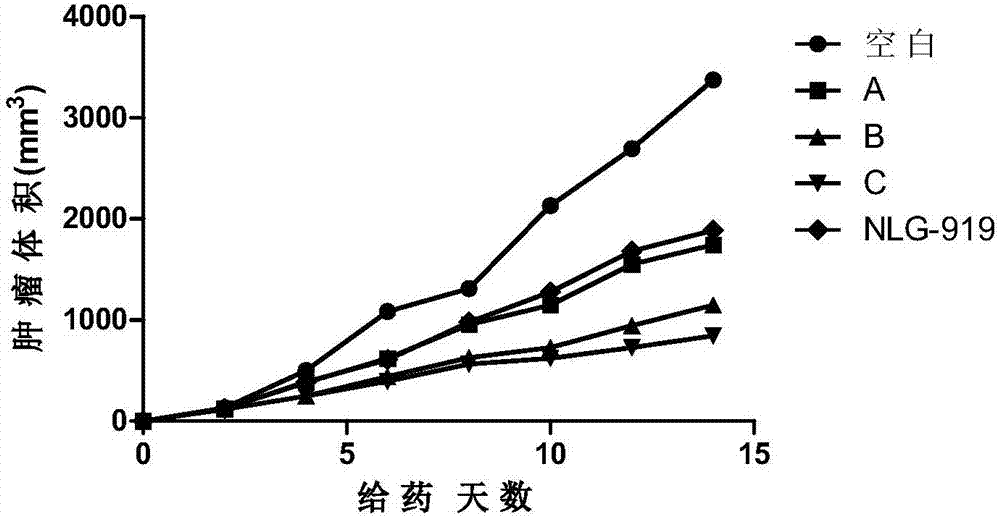
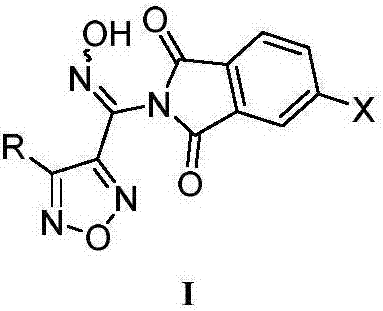
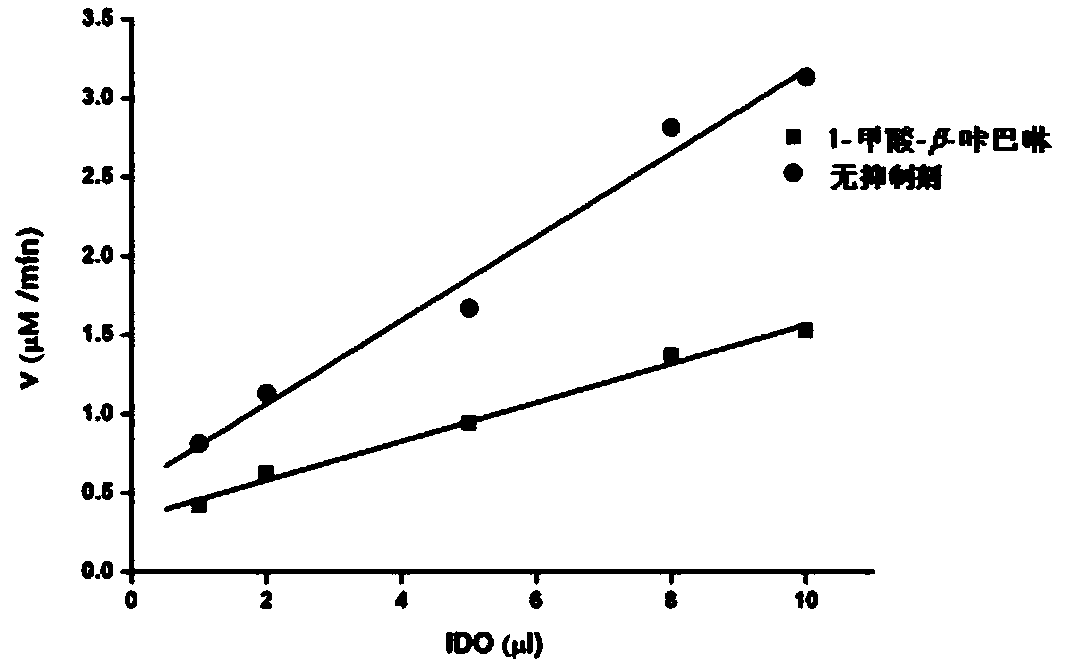
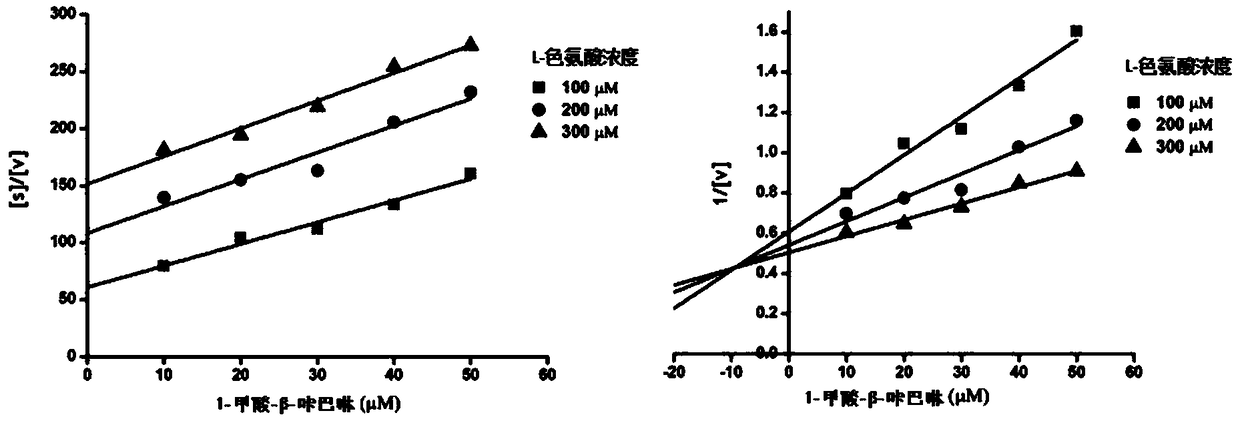
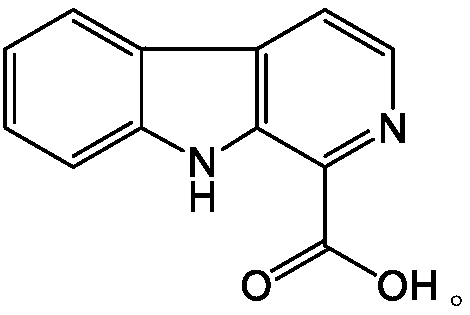
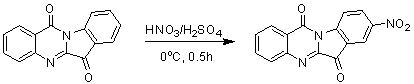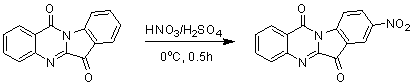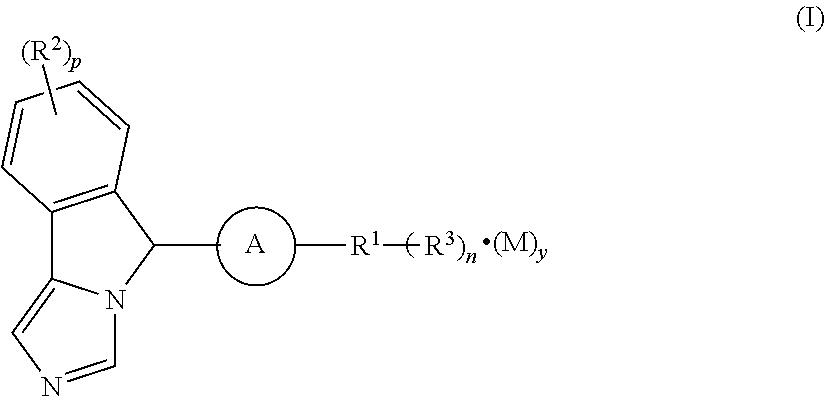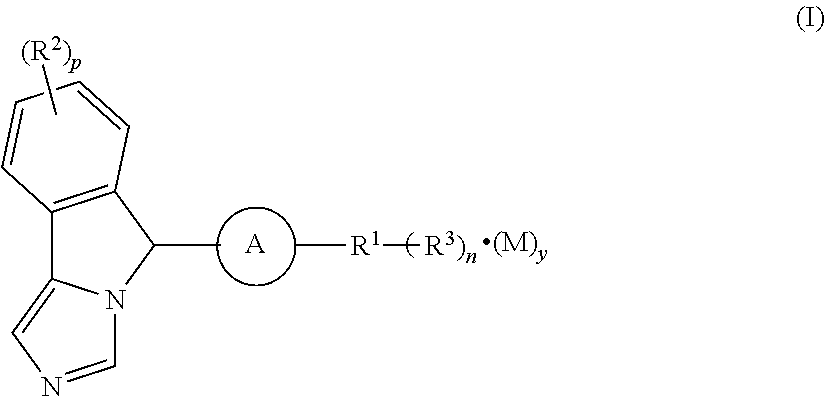Patents
Literature
64 results about "Tryptophan Metabolism Pathway" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Also, the serotonin branch of the tryptophan metabolic pathway generates NADH. Lastly, the levels of quinolinic and kynurenic acid are strongly influenced by the activity of the immune system. Therefore, decreased tryptophan metabolism may alter brain development, neuroimmune activity and mitochondrial function.
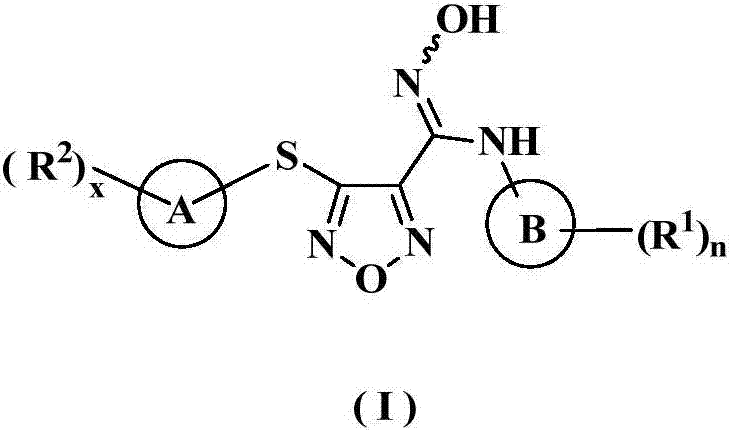
Oxadiazole derivative, preparation method therefor and use of oxadiazole derivative in medicines
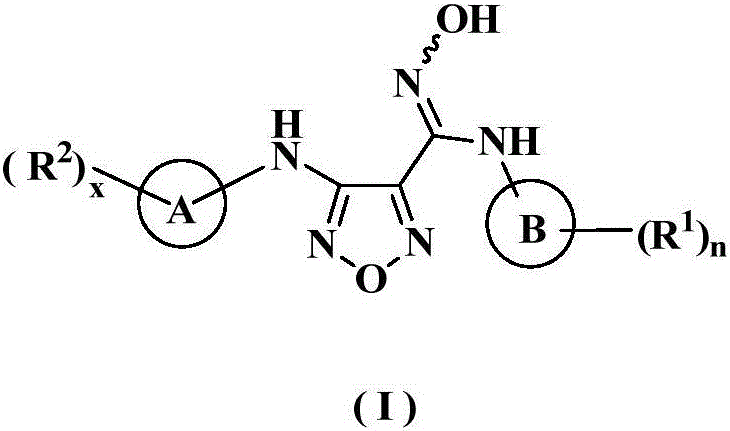
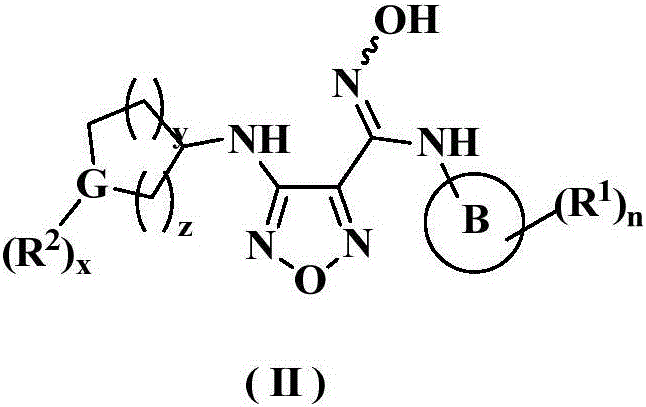
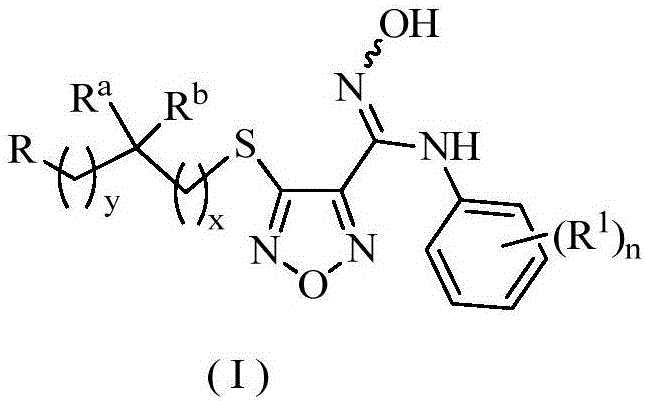
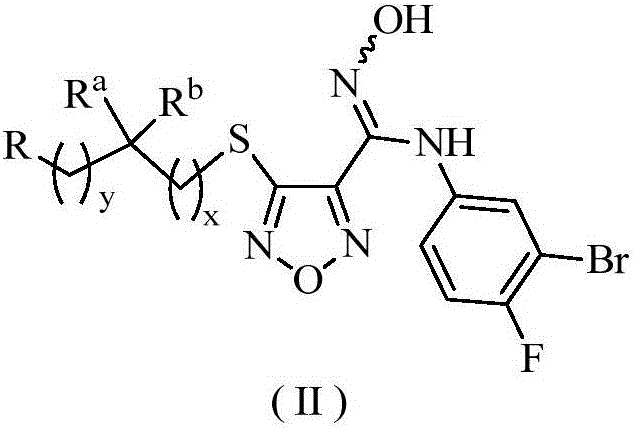
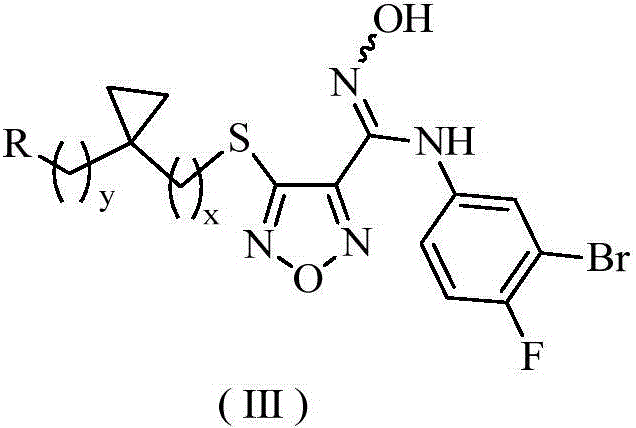
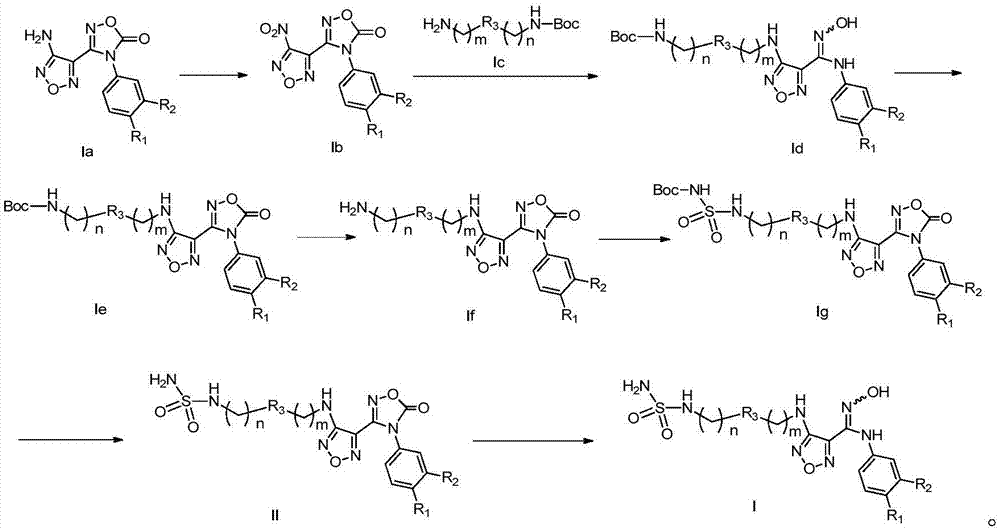
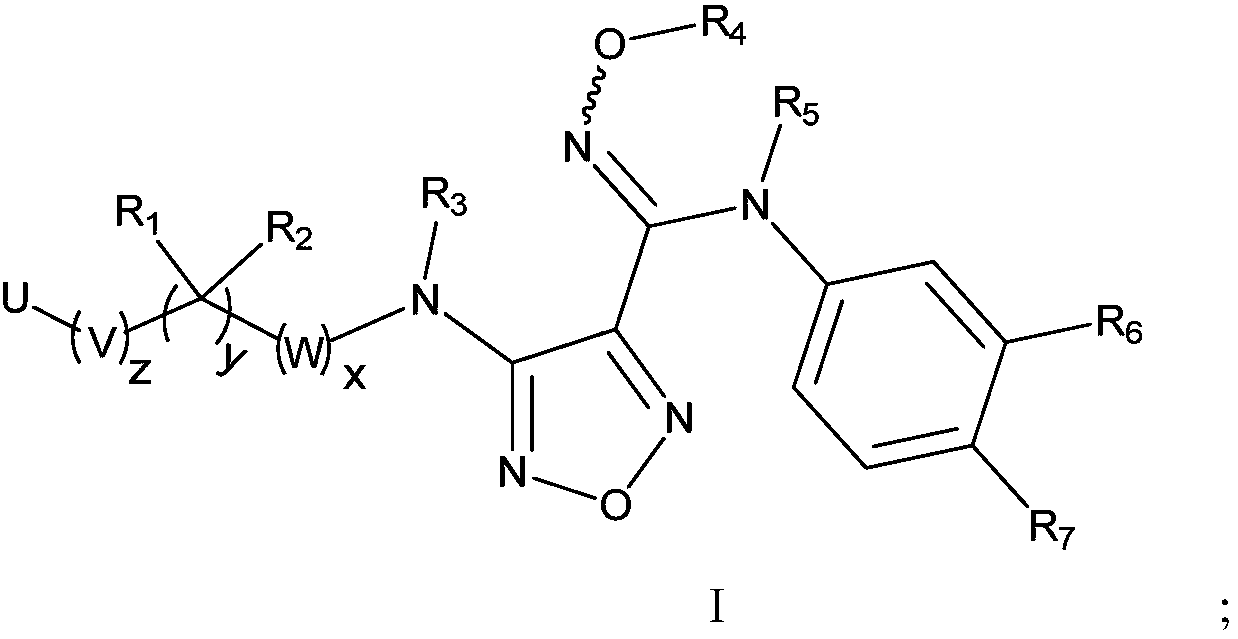
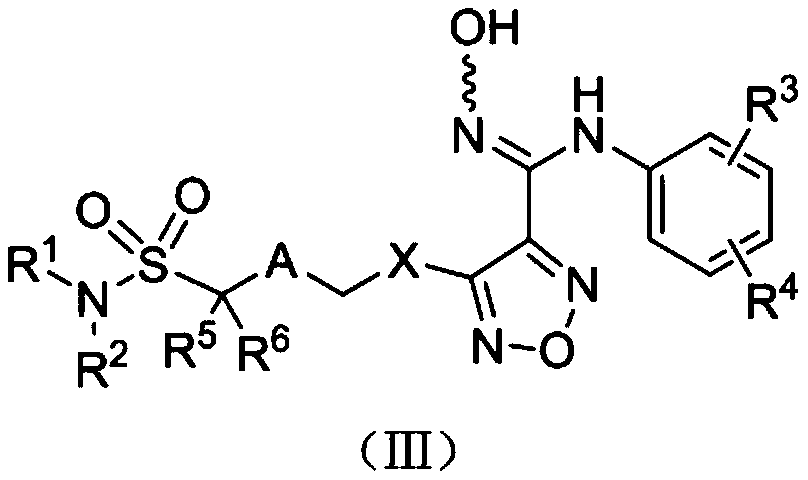
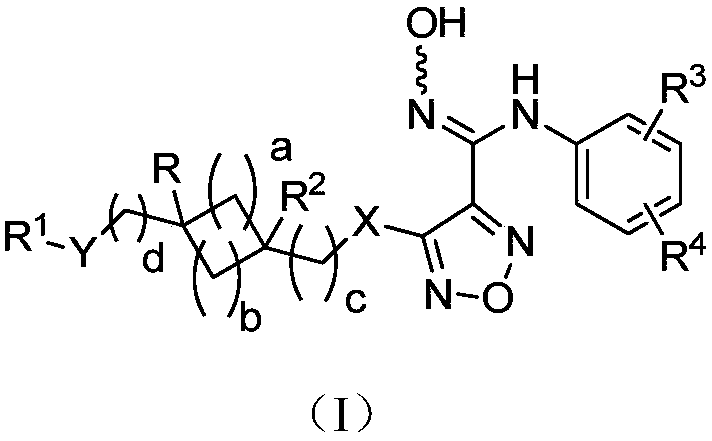
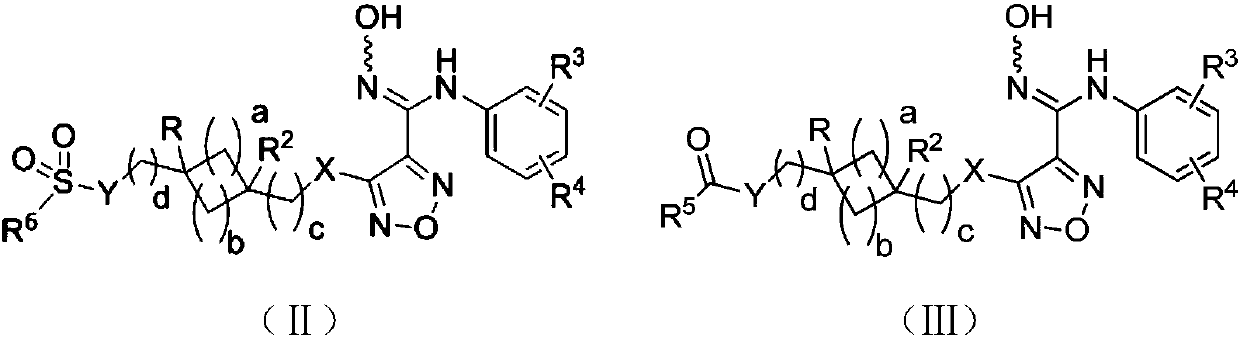
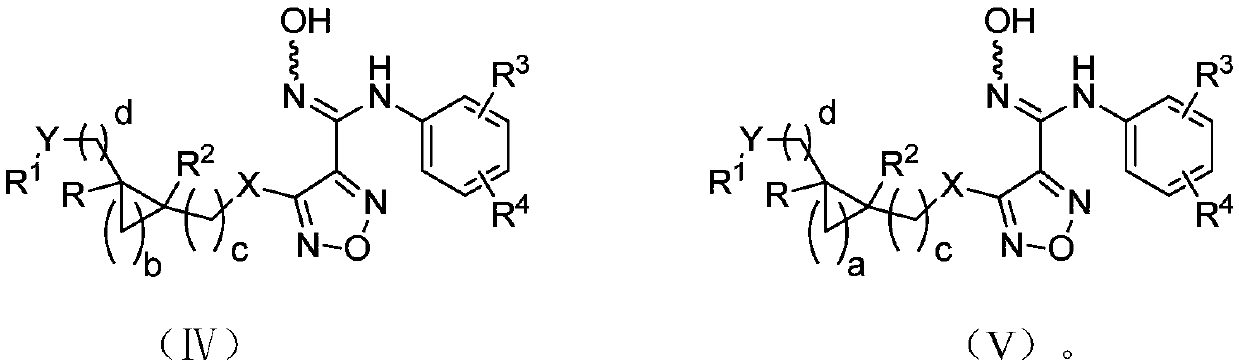
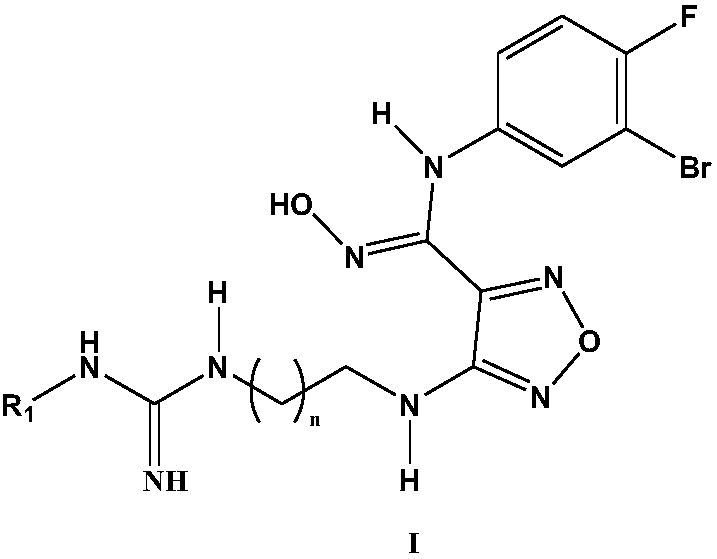
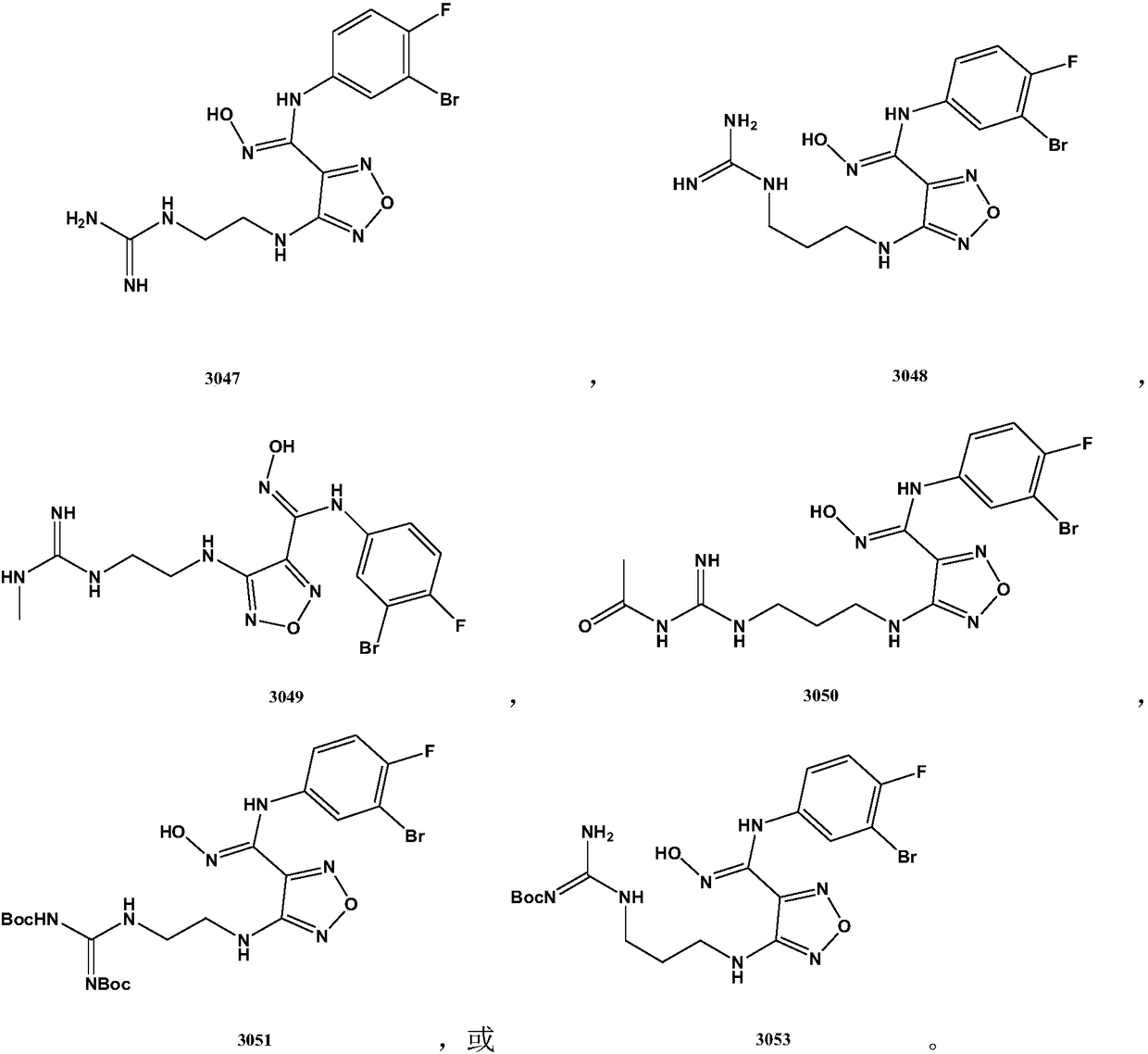
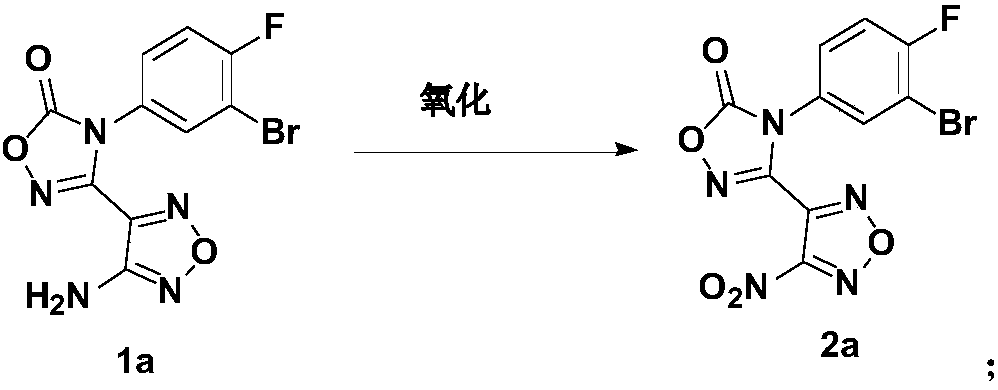
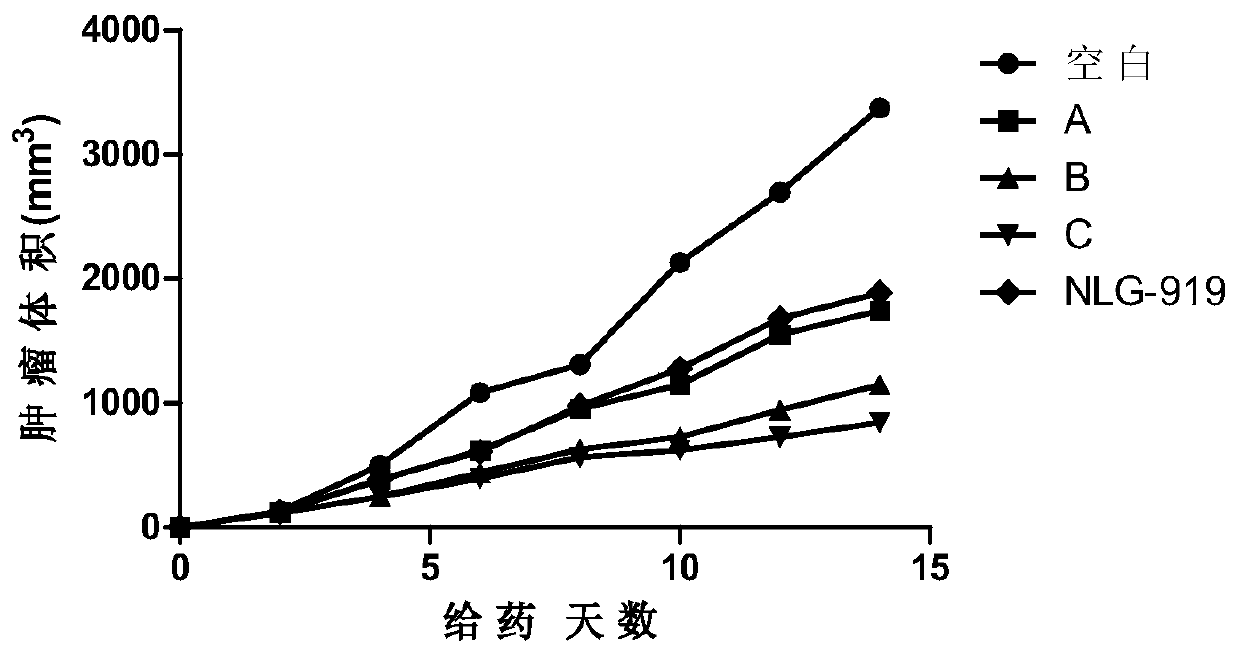
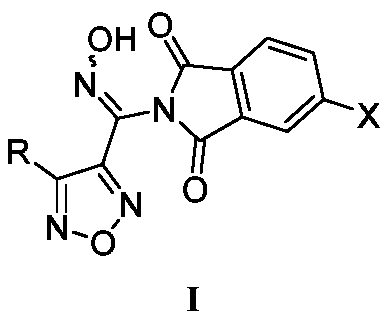
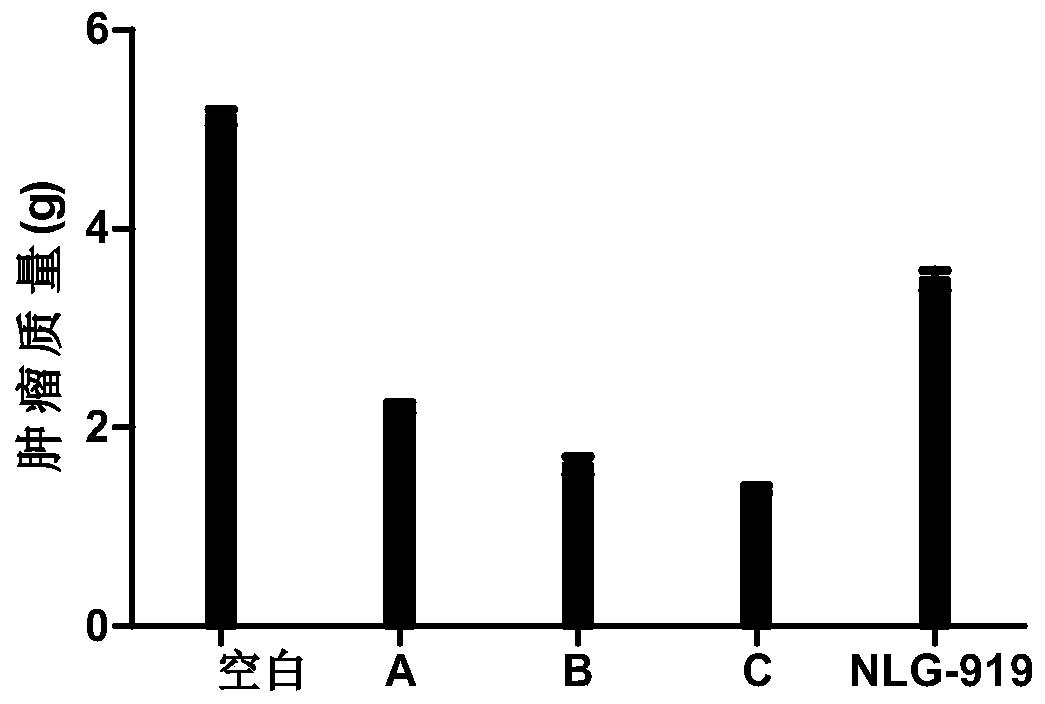
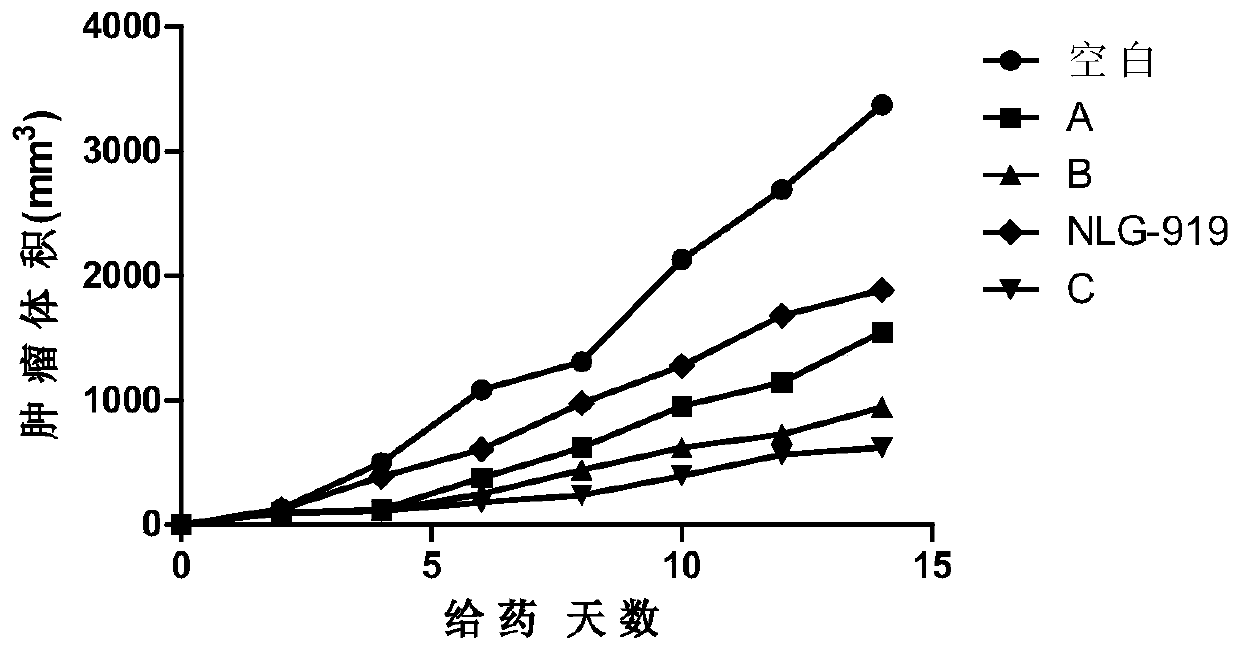
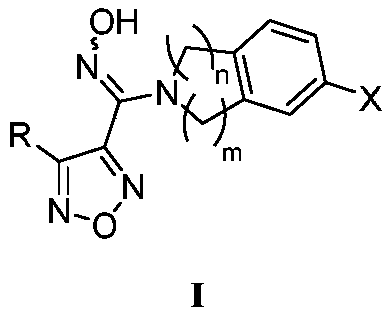
The invention relates to an oxadiazole derivative, a preparation method therefor and use of the oxadiazole derivative in medicines. Particularly, the invention relates to an oxadiazole derivative represented by a general formula (I) shown in the description, a preparation method therefor, a pharmaceutical composition containing the derivative and use of the pharmaceutical composition in preparation of drugs for preventing and / or treating diseases with IDO mediated pathological features of a tryptophan metabolism pathway, wherein the diseases comprise cancer, Alzheimer's disease, autoimmune disease, melancholia, anxiety neurosis, cataract, psychological barrier and acquired immunodeficiency syndrome. Each substituent of the general formula (I) is the same as defined in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
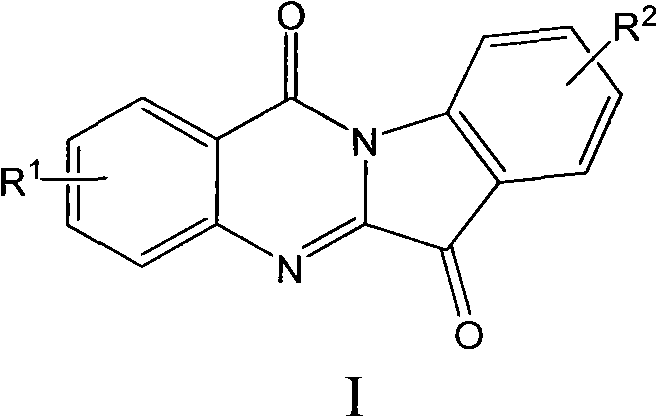
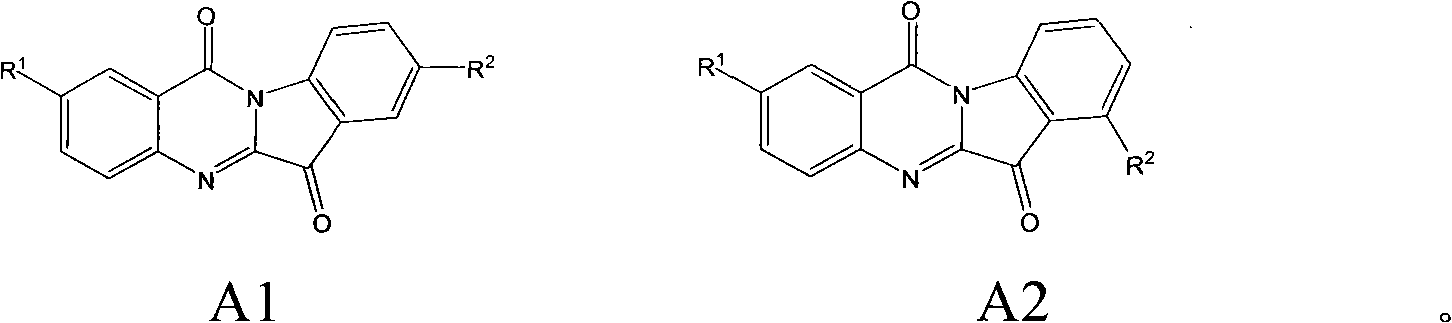
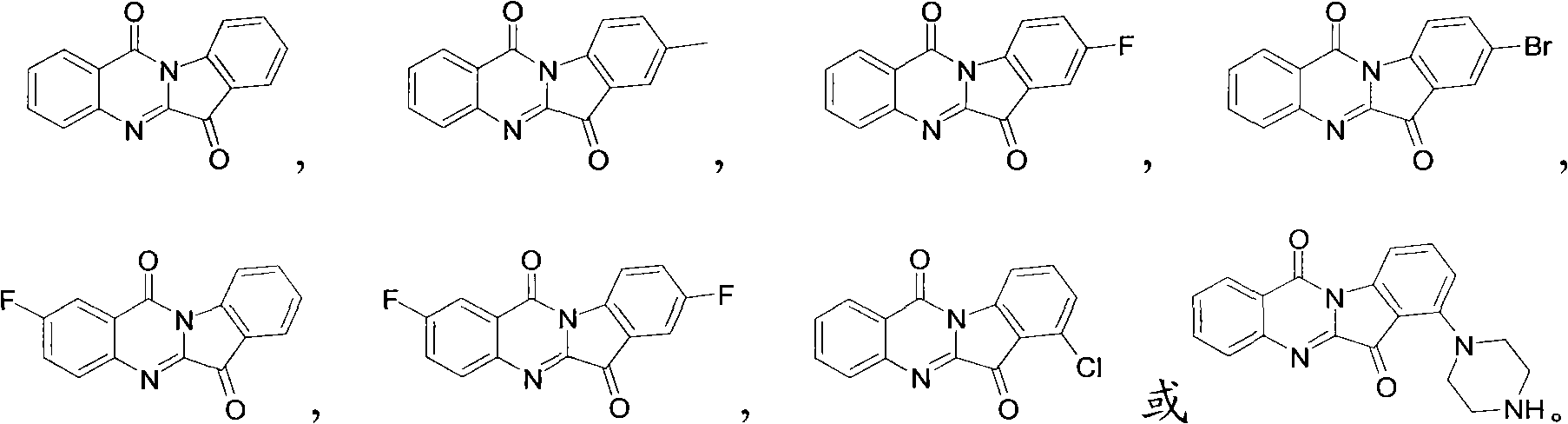
Preparation method of tryptanthrin compound and new application of tryptanthrin compound in preparing indoleamine-2,3-dioxygenase (IDO) inhibitor
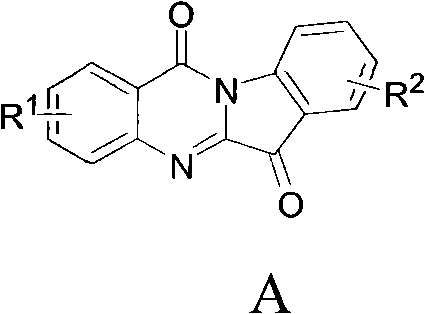
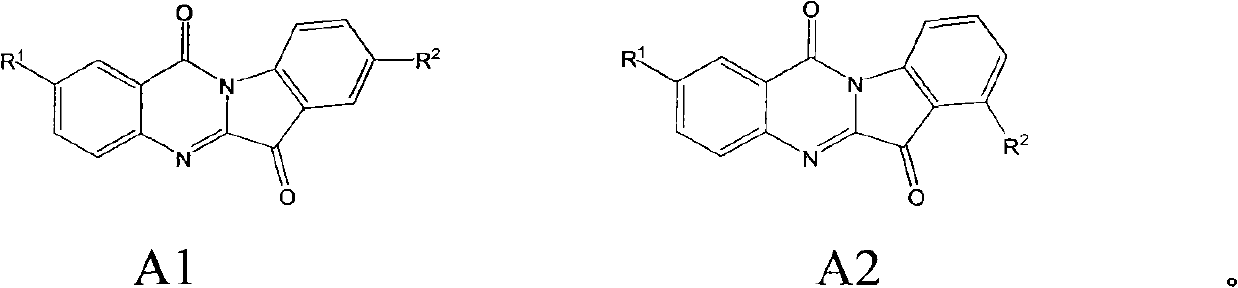
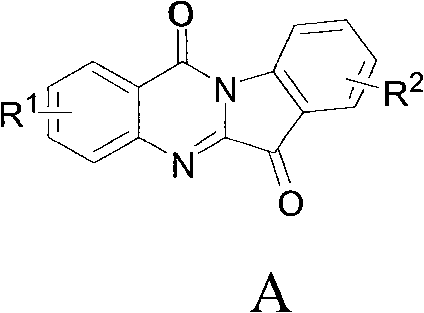
The invention relates to a preparation method of tryptanthrin compound and a new application of tryptanthrin compound in preparing indoleamine-2,3-dioxygenase (IDO) inhibitor, in particular to an application of tryptanthrin compound in preparing IDO inhibitor and in preparing medicine for preventing and / or treating diseases having pathological characteristics of tryptophan metabolic disturbance mediated by IDO. The structure of the tryptanthrin compound is shown in formula A in the description, wherein R1 and R2 are respectively and independently selected from hydrogen, nitryl, C1 to C5 alkyl, halogen or piperazinyl. The tryptanthrin compound can be used as an IDO inhibitor with high activity and is used for treating diseases having pathological characteristics of tryptophan metabolic disturbance mediated by IDO such as tumor, cancer, Alzheimer disease, autoimmune disease, cataract, mental block, tristimania, anxiety neurosis, acquired immure deficiency syndrome (AIDS) and other serious diseases, and as a medicinal resource which is very difficult to get, the tryptanthrin compound has good potential on researching and developing new medicine.
Owner:SHENYANG XINMA PHARMA
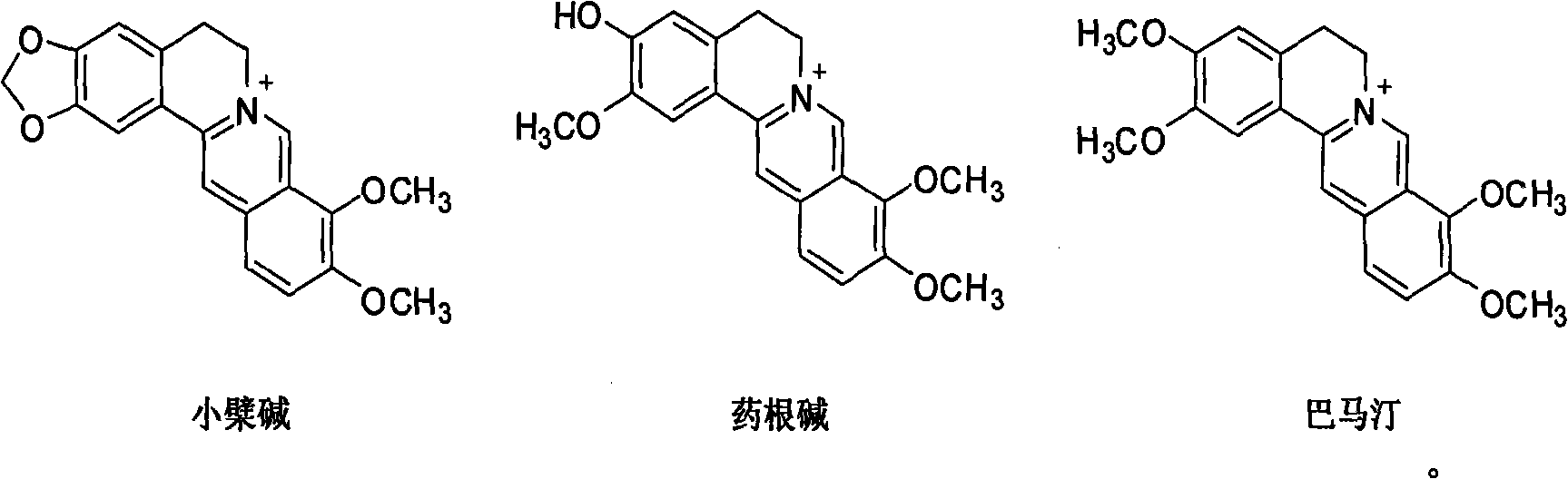
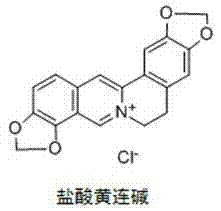
Application of berberine and derivatives thereof in preparation of indole amine 2, 3-dioxygenase inhibitor
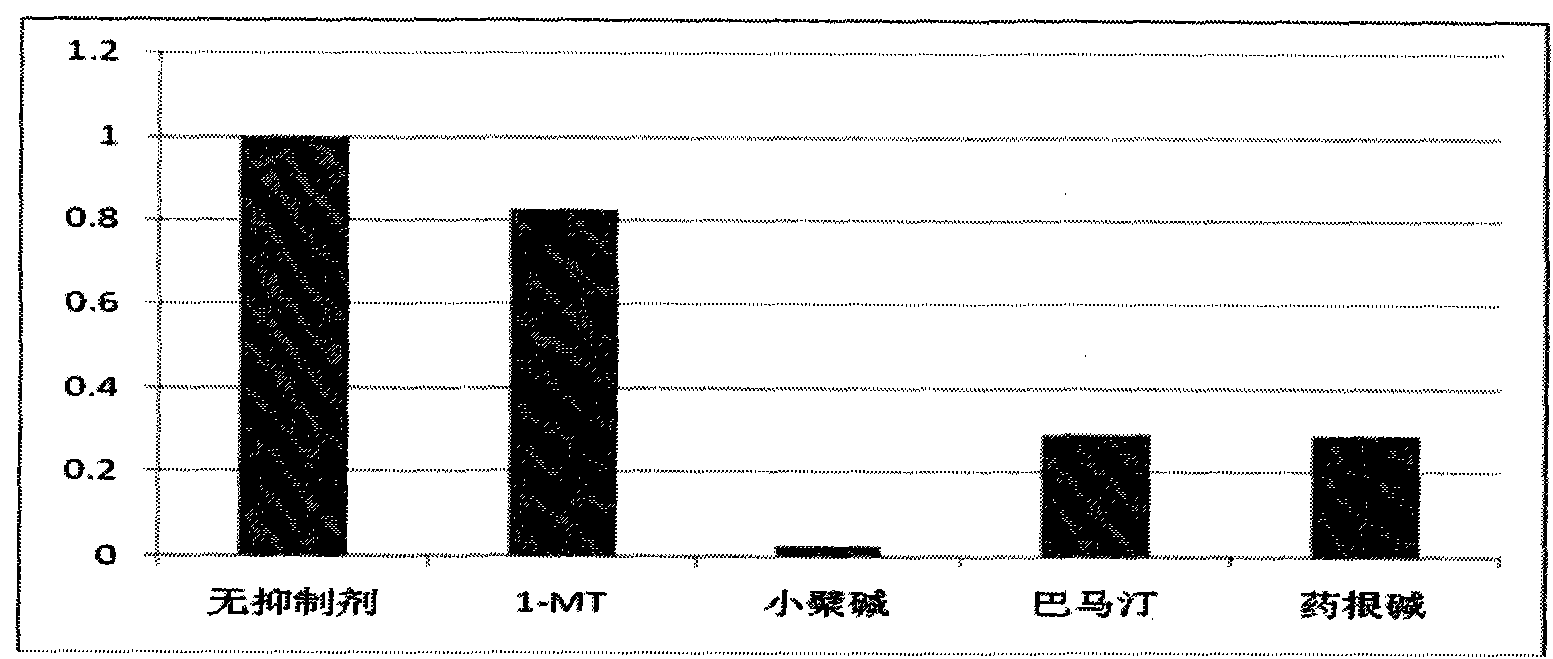
The invention relates to novel application of berberine and derivatives thereof in preparation of medicines, in particular to application of berberine and derivatives thereof in preparation of indole amine 2, 3-dioxygenase IDO inhibitor, belonging to the medicine field. According to IDO inhibition activity detection, reversible inhibition judgment, inhibitor type judgment, Ki value determination and median effective inhibition concentration IC50 determination, the results show that berberine is reversible inhibitor and inhibition constant Ki is 8muM; and jatrorrhizine hydrochloride and palmatine hydrochloride are irreversible inhibitors, and the median effective inhibition concentrations IC50 thereof are respectively 123muM and 126muM. When the berberine and the derivatives thereof disclosed in the invention are used as IDO inhibitors, the application prospect is wide and the IDO inhibitors can be used for treating serious diseases such as cancers, AIDS, Alzheimer diseases, tristimania, cataract and the like with pathological feature of IDO-mediated tryptophan metabolism.
Owner:FUDAN UNIV
Novel indoleamine-2,3-dioxygenase inhibitor as well as preparation method and application thereof
ActiveCN102532144AImprove solubilityImprove pharmacological activityOrganic active ingredientsSenses disorderImmunologic disordersAutoimmune condition
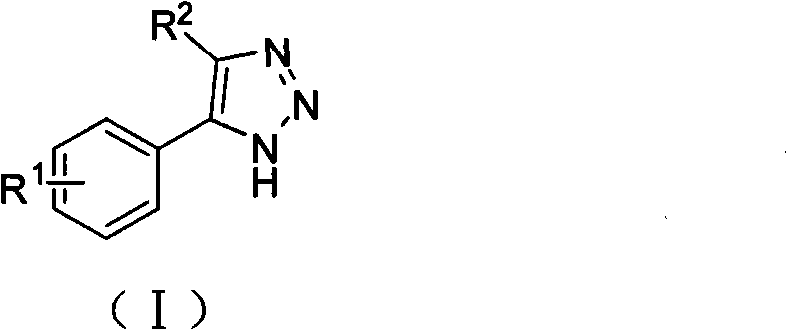
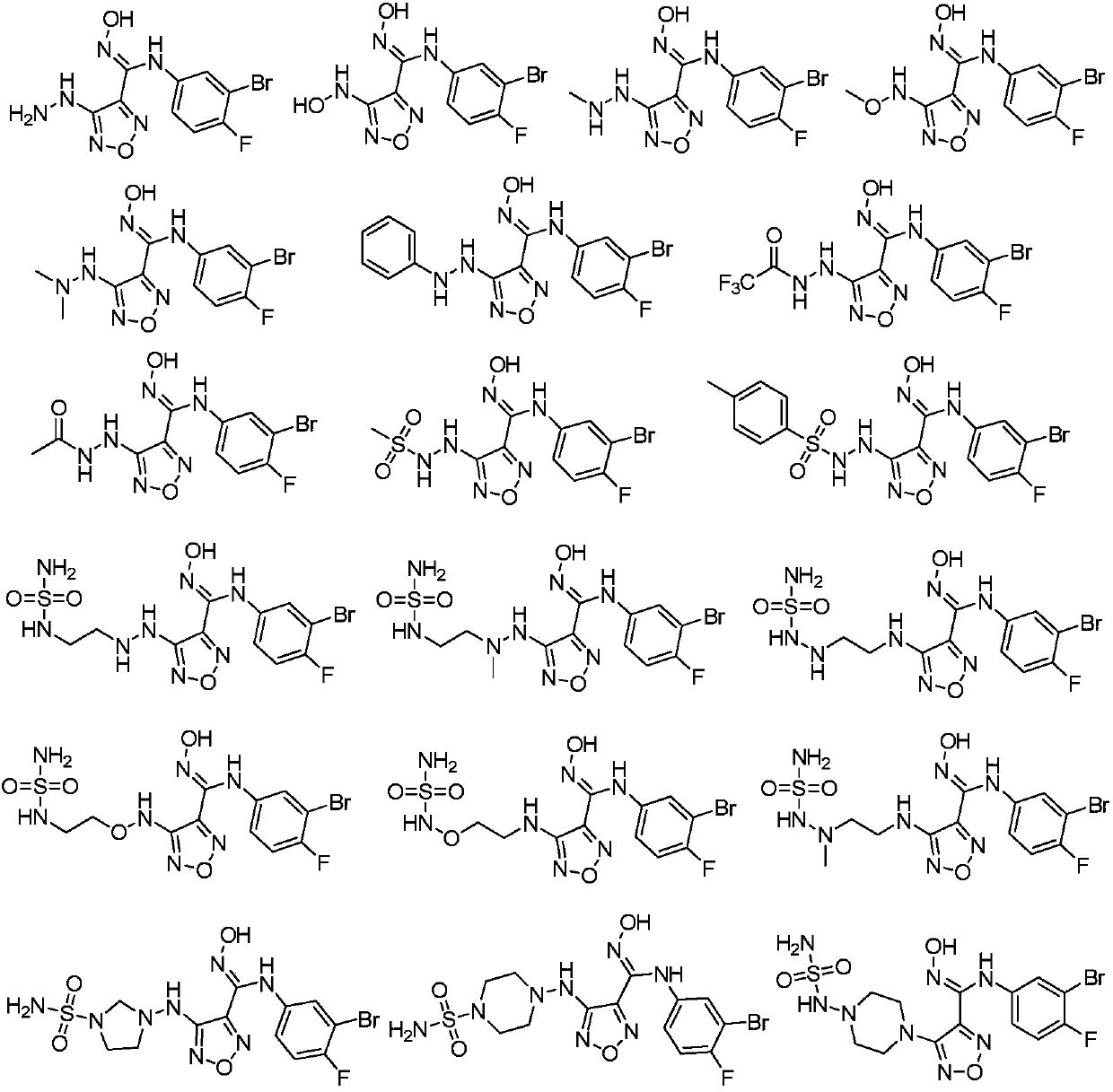
The invention relates to a novel indoleamine-2,3-dioxygenase (IDO) inhibitor, as well as a preparation method and an application thereof. The inhibitor is compounds of a formula I or pharmaceutically-acceptable salt, solvate, polymorph, enantiomer or racemic mixture thereof, wherein R1 or R2 is independently selected from H, C1-C5 alkyl, halogen and a substituent of a formula II and either of R1 and R2 is the substituent of the formula II; R3 is an aryl group; and n is equal to 0, 1, 2 or 3. Compared with the know IDO inhibitors, the novel IDO inhibitor has significant inhibitory effect on IDO and can be used for treatment of diseases having IDO-mediated tryptophan metabolic pathway pathological features, such as tumors, cancers, Alzheimer's disease, autoimmune diseases, cataract, psychological disorders, depression and / or anxiety. The preparation method of the novel IDO inhibitor has the advantages of being convenient in operation, having mild reaction conditions and reducing solvent consumption and pollution, thereby facilitating industrial production.
Owner:SHENYANG XINMA PHARMA
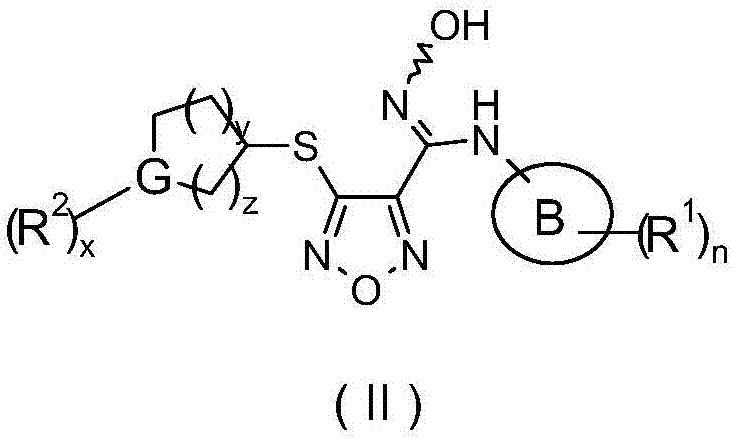
Oxadiazole derivative, preparing method of oxadiazole derivative and application of oxadiazole derivative to medicines
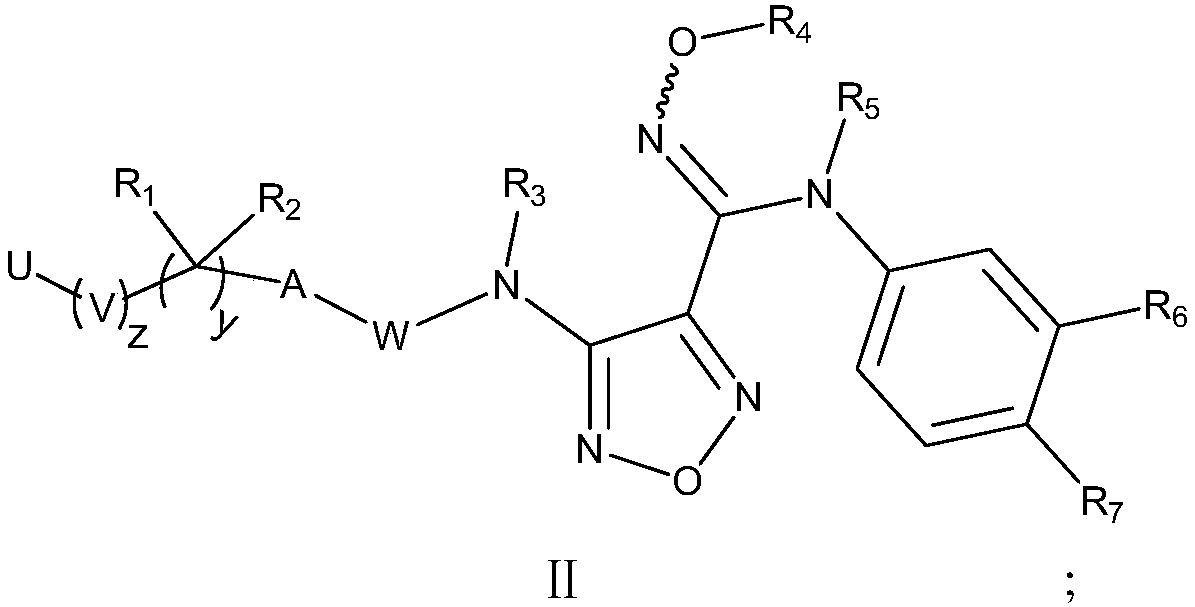
The invention relates to an oxadiazole derivative, a preparing method of the oxadiazole derivative and application of the oxadiazole derivative to medicines, in particular to an oxadiazole derivative shown in the general formula (1), a preparing method of the oxadiazole derivative, a medicine composition comprising the derivative and application of the oxadiazole derivative to treatment of diseases with the tryptophan metabolic pathway pathological feature mediated by IDO. The diseases include the cancer, the alzheimer disease, the autoimmune disease, the depression, the anxiety disorder, the cataract, the psychological disorder and the AIDS. Definition of substituent groups in the general formula (1) is the same with the definition of the substituent groups in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
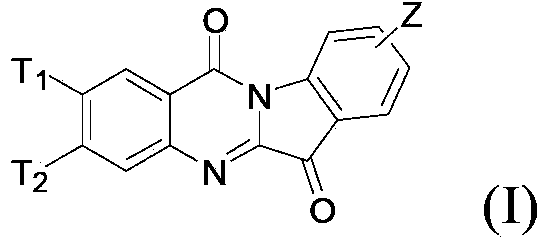
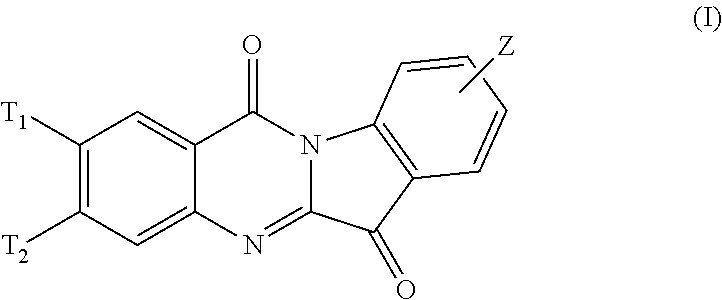
N-alkyl tryptanthrin derivative, as well as preparation method and application thereof
ActiveCN103570726AHigh IDO inhibitory activityRealize industrial productionOrganic active ingredientsSenses disorderDiseaseTryptophan
The invention discloses an N-alkyl tryptanthrin derivative, as well as a preparation method and an application thereof. The structure of the N-alkyl tryptanthrin derivative is as shown in a formula I, wherein T1, T2 and Z are as defined in description and claims. The N-alkyl tryptanthrin derivative disclosed by the invention can be used as a high-activity IDO (indoleamine 2, 3-dioxygenase) inhibitor, and can be used for preparing medicaments for preventing and / or treating diseases with pathological characteristics of IDO-mediated tryptophan metabolic ways.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Use of tryptanthrin compound as indoleamine 2,3-dioxygenase (IDO) inhibitor
InactiveCN103054870AExcellent inhibitory effectOrganic active ingredientsNervous disorderDiseaseInhibition constant
The invention belongs to the medicinal field, and concretely relates to a use of a 8-nitrotryptanthrin compound as an IDO inhibitor. The 8-nitrotryptanthrin compound is a reversible IDO inhibitor, has an inhibition constant Ki of 0.054muM, has in-vitro and cell-based median effective inhibition concentrations IC50 of 0.103muM and 1.80*10<-5>muM respectively, and has an inhibition effectiveness obviously better than a present inhibitor 1-methyltryptophan (Ki of 34muM and IC50 of 340muM). 8-nitrotryptanthrin disclosed in the invention can effectively lower the abnormally-increasing IDO activity in a tumor animal model as the IDO inhibitor, and also has tumor treatment effects comprising tumor growth delaying, tumor volume reduction and in-vitro tumor cell killing. 8-nitrotryptanthrin disclosed in the invention has a wide application prospect, and can be used for treating serious diseases having the IDO mediated tryptophan metabolism approach pathology characteristics, such as cancers, the Alzheimer disease, tristimania, cataract and the like as the IDO inhibitor.
Owner:FUDAN UNIV
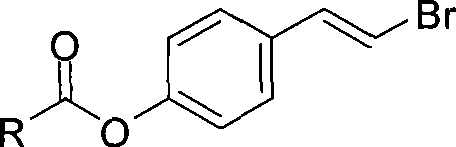
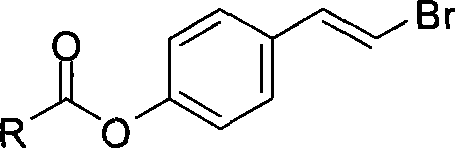
IDO restrainer containing (E)-4-(beta-bromo vinyl)benzoyloxy structure
The invention belongs to the technical field of new drug synthesis, and in particular relates to an IDO inhibitor containing (E)-4-(beta-bromovinyl)phenoxy acyl structure, as well as a preparation method thereof. The preparation method comprises the following steps: solvent benzene, (E)-4-(beta-bromovinyl) phenol, carboxylic acid and p-dimethylaminopyridine are added to a flask respectively and magnetically stirred for 5 to 20 minutes at room temperature; then N, N'-dicyclohexylcarbodiimide is added and reacts for 2 to 24 hours at room temperature; after the reaction is completed, the solvent benzene is steamed off after decompression; residue is subjected to column chromatography separation and purification by taking ethyl acetate / petroleum ether as leacheate, and then a needed product can be obtained, wherein the molar ratio of the solvent benzene to the (E)-4-(beta-bromovinyl) phenol is 50-200:1; the molar ratio of the carboxylic acid to the (E)-4-(beta-bromovinyl) phenol is 1-1.5:1; the molar ratio of the p-dimethylaminopyridine to the (E)-4-(beta-bromovinyl) phenol is 0.1-1.5:1; and the molar ratio of the N, N'-dicyclohexylcarbodiimide to the (E)-4-(beta-bromovinyl) phenol is 1-1.2:1. The method takes the (E)-4-(beta-bromovinyl) phenol as a synthesis building block and obtains a series of the novel IDO inhibitors containing the (E)-4-(beta-bromovinyl)phenoxy acyl structure through esterification reaction, and the IDO inhibitors can be used for treating the diseases with the pathological features of IDO mediated tryptophan metabolic pathway.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
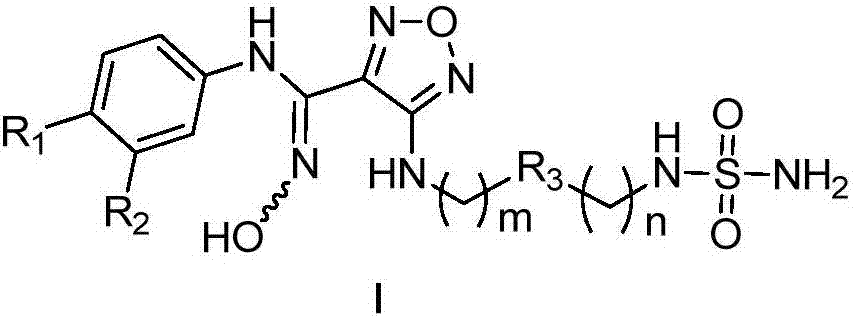
Oxadiazoles derivative, preparation method and medical application thereof
ActiveCN107033097AOrganic active ingredientsSenses disorderImmunologic disordersAutoimmune thyroid disease
The invention relates to an oxadiazoles derivative, a preparation method and a medical application thereof, in particular to the oxadiazoles derivative of general formula 1, the preparation method, a drug composition containing the oxadiazoles derivative, and the application thereof in preparing medicines which can prevent and / or cure diseases with an IDO mediated tryptophan metabolic pathway histopathologic characteristic. The diseases comprise cancer, alzheimer disease, autoimmune disease, depression, anxiety disorder, cataract, psychologic obstacle and HIV disease. Each substituent of the general formula (1) is same with the definition in the description.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Compound capable of inhibiting IDO (indoleamine 2, 3-dioxyenase), and preparation method and application of compound
ActiveCN106967005AStrong inhibitory activityOrganic active ingredientsSenses disorderHalogenHydrogen atom
The invention discloses a compound capable of inhibiting IDO (indoleamine 2, 3-dioxyenase), and a preparation method and application of the compound. The structural general formula is shown as below; - is selected from a mixture of a cis-isomer, a trans-isomer or a cis-trans-isomer; R1 and R2 are independently selected from any one of hydrogen atoms, halogen, alkyl, alkoxy or halogenated alkyl; R3 is selected from any one of cyclopentyl, cyclohexyl, piperazinyl or piperidyl; the substitution position of the R3 is (1, 2), (1, 3) or (1, 4); m and n are selected from integers of 0-5 respectively. The compound capable of inhibiting the IDO has high inhibition activity on the IDO, can be used for preparing IDO inhibitors to prevent and / or treat diseases with pathological features of tryptophan metabolic pathways mediated by the IDO, and has quite good application prospect.
Owner:SHANGHAI JOYU PHARMATECH LTD
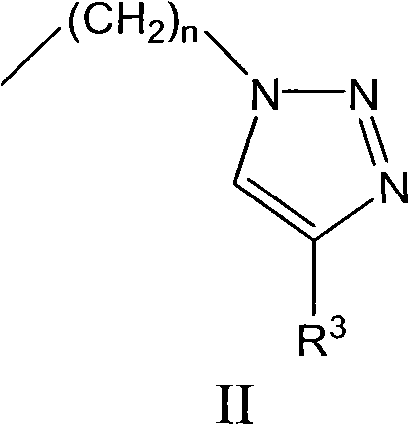
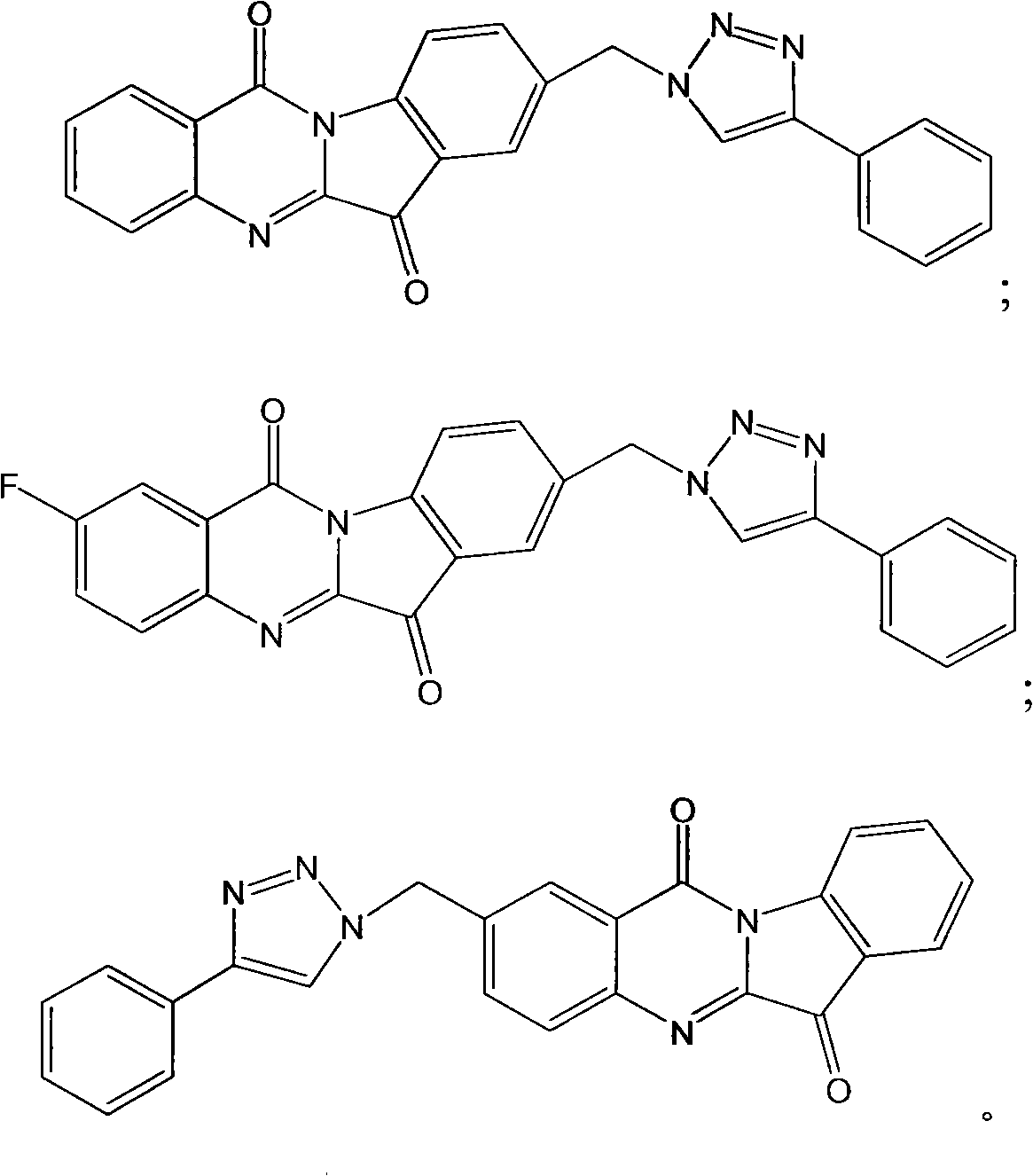
1, 2, 3-triazole compound and application thereof in preparing indoleamine 2, 3-dioxygenase inhibitor
InactiveCN101786993APromote decompositionLow toxicitySenses disorderNervous disorderDiseaseTryptophan
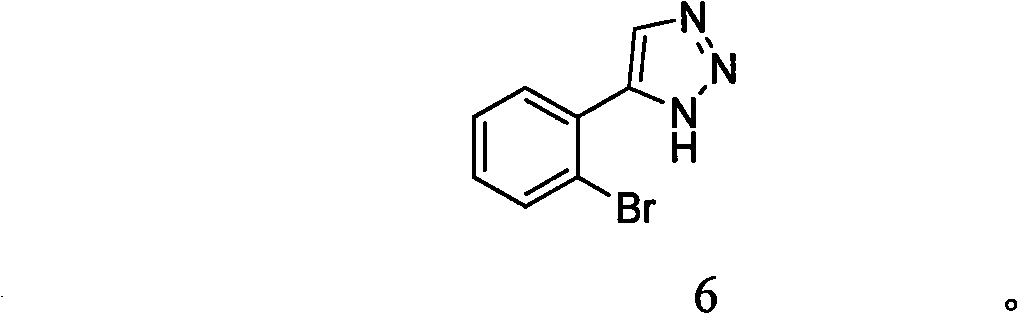
The invention belongs to the field of pharmaceutical chemistry and relates to a 1, 2, 3-triazole compound, comprising 4-(2-bromophenyl)-1H-1, 2, 3-triazole and 4-(2-chlorphenyl)-1H-1, 2, 3-triazole. The result of an inhibitory activity test of indoleamine 2, 3-dioxygenase indicates that the disclosed 1, 2, 3-triazole compound used as the indoleamine 2, 3-dioxygenase inhibitor has broad prospect of application and can be applied to the treatment of diseases with indoleamine 2, 3-dioxygenase-mediated tryptophan metabolic pathway pathological characteristics, including cancer, AIDS (Acquired Immure Deficiency Syndrome), Alzheimer disease, tristimania, cataract and other critical diseases.
Owner:FUDAN UNIV
Compound used as IDO (Indoleamine-2,3-dioxygenase) regulator and application thereof
The invention belongs to the technical field of medicines and in particular discloses a compound shown as a formula I or a formula II. Each symbol is defined as claims. The compound shown as the formula I or the formula II, provided by the invention, can be used as an IDO (Indoleamine-2,3-dioxygenase) regulator; the invention specifically discloses application of the compound to preparation of a medicine for treating IDO-mediated diseases with tryptophan metabolic pathway pathologic features. The formula I and the formula II are shown in the description.
Owner:TETRANOV PHARMA CO LTD
Preparation method of tryptanthrin compound and new application of tryptanthrin compound in preparing indoleamine-2,3-dioxygenase (IDO) inhibitor
The invention relates to a preparation method of tryptanthrin compound and a new application of tryptanthrin compound in preparing indoleamine-2,3-dioxygenase (IDO) inhibitor, in particular to an application of tryptanthrin compound in preparing IDO inhibitor and in preparing medicine for preventing and / or treating diseases having pathological characteristics of tryptophan metabolic disturbance mediated by IDO. The structure of the tryptanthrin compound is shown in formula A in the description, wherein R1 and R2 are respectively and independently selected from hydrogen, nitryl, C1 to C5 alkyl, halogen or piperazinyl. The tryptanthrin compound can be used as an IDO inhibitor with high activity and is used for treating diseases having pathological characteristics of tryptophan metabolic disturbance mediated by IDO such as tumor, cancer, Alzheimer disease, autoimmune disease, cataract, mental block, tristimania, anxiety neurosis, acquired immure deficiency syndrome (AIDS) and other serious diseases, and as a medicinal resource which is very difficult to get, the tryptanthrin compound has good potential on researching and developing new medicine.
Owner:SHENYANG XINMA PHARMA
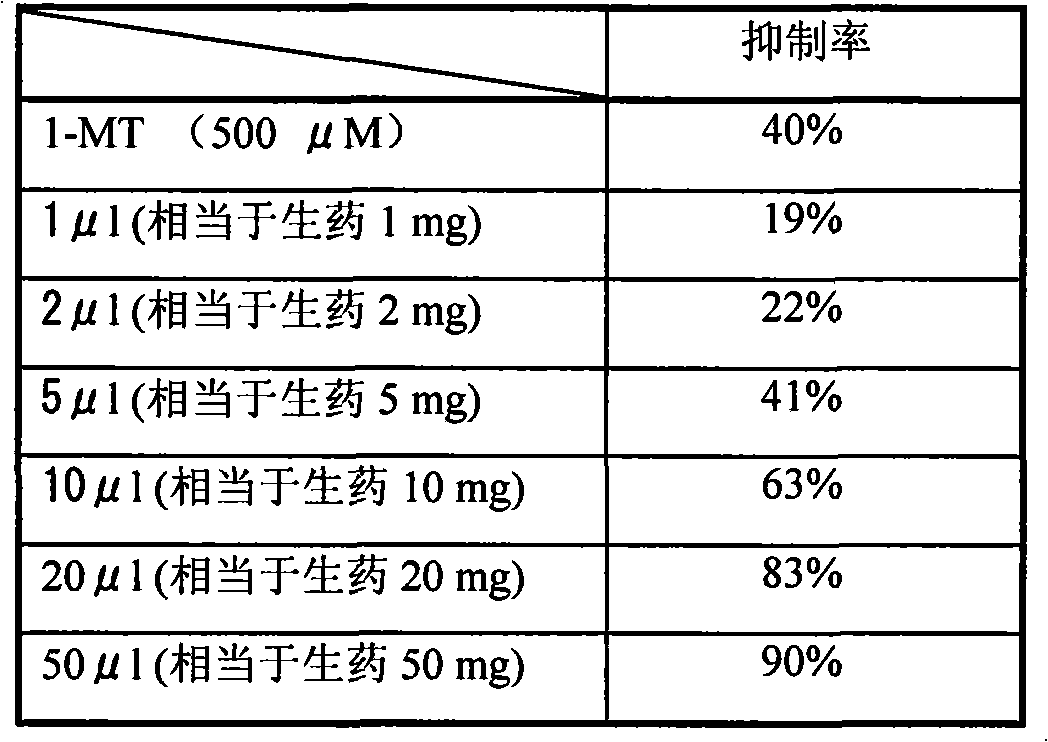
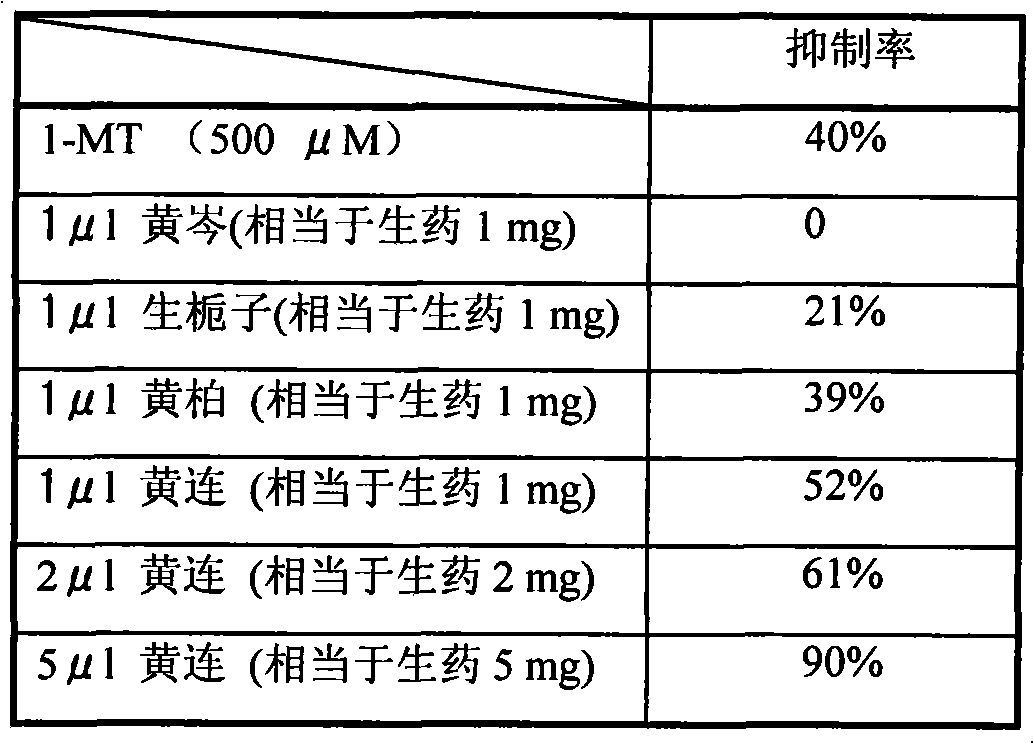
Application of golden thread detoxication decoction as compound preparation in preparing IOD active medicaments
InactiveCN101837066AAvoid missingOrganic active ingredientsSenses disorderBaical Skullcap RootTryptophan Metabolism Pathway
The invention belongs to the field of traditional Chinese medicines and relates to new medicament application of a golden thread detoxication decoction as a compound preparation, in particular to application of the golden thread detoxication decoction as the compound preparation in preparing IOD active medicaments. In the invention, an in-vitro IOD activity detecting system is established by using recombined human IDO enzyme, and experimental results indicate that the golden thread detoxication prepared from Chinese medicinal crops of golden thread, baical skullcap roots, golden cypress and Fructus Gardeniae as well single medicine and single chemical components of the composition thereof have IDO inhibition activities. The golden thread detoxication decoction and the single medicine of the composition thereof can be used for inhibiting IDO and can be used as a medicament raw material for preparing medicaments for treating or preventing diseases with the pathological characteristics of IDO-mediated tryptophane metabolic pathways.
Owner:FUDAN UNIV
Vitamin K3 derivatives serving as IDO inhibitor
InactiveCN101830792ARaw materials are easy to getSimple and fast operationOrganic active ingredientsSenses disorderImmunologic disordersDisease
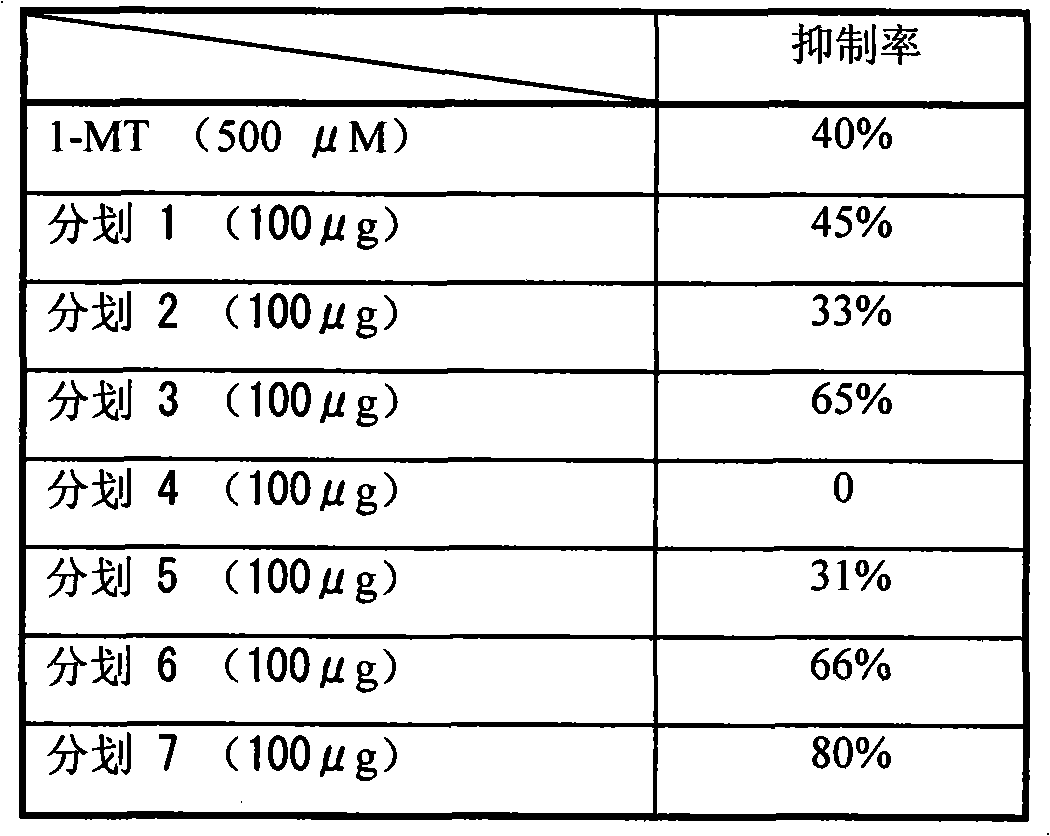
The invention belongs to the technical fields of medicaments and intermediate synthesis, and in particular relates to vitamin K3 derivatives and a preparation method thereof. The 1,4-naphthoquinone derivatives are conveniently synthesized by using 2-bromine-1,4-naphthoquinone as raw materials through one-step reaction. The vitamin K3 derivatives synthesized by the method contain vitamin K3 structures and can be used as medicaments and important synthesized intermediates. The method for preparing the vitamin K3 derivatives has the characteristics of simple operation, mild condition, low cost, high yield, and the like, has wide application prospect, and is easy for industrialized production. An IDO inhibitor synthesized by the method and containing the vitamin K3 structure can be applied to the treatment of diseases with IDO-mediated tryptophan metabolic pathway pathologic features, wherein the diseases at least comprise tumor diseases, cancers, Alzheimer, autoimmune diseases, cataracts, mood disorders, depression, and anxiety disorder.
Owner:CONJOIN PHARM SUZHOU CO LTD
Fused imidazole compounds with indoleamine 2,3-dioxygenase inhibition activity
The invention relates to fused imidazole compounds, and a preparation method and application thereof. The structure of the compounds is shown in a general formula I, wherein the definitions of each group are as described in the specification. The compounds are capable of selectively inhibiting indoleamine 2,3-dioxygenase (IDO). The compounds provided by the invention can be used as IDO inhibitorsfor the treatment and / or prevention of diseases with the pathological characteristics of IDO mediated tryptophan metabolism pathways, such as cancer, eye diseases, autoimmune diseases, psychological disorders, depression, anxiety and other diseases.
Owner:SHENZHEN CHIPSCREEN BIOSCIENCES CO LTD
Targeted indoleamine-2,3-dioxygenase 1 nitrogen mustard inhibitor as well as preparation method and application thereof
InactiveCN107987031AStrong inhibitory activityBroad-spectrum antitumor activity in vitroOrganic active ingredientsOrganic chemistryAbnormal tissue growthDisease
The invention discloses a targeted indoleamine-2,3-dioxygenase 1 nitrogen mustard inhibitor as well as a preparation method and application thereof. A structure of the inhibitor is shown as a generalformula I, wherein definition of R is as shown in the claims and specification. Pharmacological experiments prove that the compound disclosed by the invention has excellent IDO1 inhibitory activity and has broad-spectrum in-vitro antitumor activity. In-vivo experiments prove that the compound disclosed by the invention can achieve effects of down-regulating the in-vivo IDO1 activity and obviouslydelaying tumor growth, and can be applied to preparing IDO1-mediated medicines for tumor diseases with pathological features in a tryptophan metabolism pathway. The structural formula is as shown in the specification.
Owner:EAST CHINA UNIV OF SCI & TECH
Indoleamine-2,3-dioxygenase inhibitor, preparation method and uses thereof
PendingCN110128367AEnhanced inhibitory effectImprove pharmacokineticsOrganic active ingredientsSenses disorderDiseaseTryptophan
The present invention provides a compound represented by a formula (I), and relates to a pharmaceutical composition containing the compound represented by the formula (I), and uses of the compound inpreparation of indoleamine-2,3-dioxygenase (IDO) inhibitor drugs. According to the present invention, the prepared compound has obvious inhibitory effect on IDO protease, and is metabolically stable in vivo; and the compound or the pharmaceutical composition thereof can be used for the preparation of IDO inhibitor drugs, and can further be used for the preparation of drugs for preventing and / or treating diseases having pathological characteristics of IDO-mediated tryptophan metabolism pathway.
Owner:HINOVA PHARM INC
Indoleamine-2,3-dioxygenase inhibitor as well as preparation method and application thereof
PendingCN109988120AGood inhibitory effectMetabolic stability in the bodyOrganic active ingredientsSenses disorderDiseaseOxygenase
The invention provides a compound as shown in a formula (I). The invention also relates to a pharmaceutical composition containing the compound of the formula (I) and application of the compound to preparation of an indoleamine-2,3-dioxygenase (IDO) inhibitor drug. The compound prepared by the method of the invention has an obvious inhibiting effect on IDO protease and is stable in metabolism in vivo. The compound or a pharmaceutical composition thereof provided by the invention can be used for preparing the IDO inhibitor drug, and can also be used for preparing medicines for preventing and / ortreating diseases with pathological characteristics of an IDO-mediated tryptophan metabolic pathway.
Owner:HINOVA PHARM INC
Carbamidine derivative
ActiveCN108794423AImprove learning and memory impairmentIncrease spaceAntibacterial agentsOrganic active ingredientsDiseaseFuran
The invention relates to the field of drugs, in particular to a carbamidine derivative containing a furan structure and a composition of the carbamidine derivative. The derivative and the compositionthereof can be used for preparing drugs for treating a pathologic characteristic disease with an indoleamine 2,3-dioxygenase mediated tryptophan metabolic pathway. The invention further relates to a method for preparing the derivative and an intermediate thereof.
Owner:LUNAN PHARMA GROUP CORPORATION
Phthalimide indoleamine-2,3-dioxygenase 1 inhibitor and use thereof
The invention relates to the field of medicinal chemistry, in particular to an IDO1 (indoleamine-2,3-dioxygenase 1) inhibitor (I) of a phthalimide structure and a preparation method, wherein X and R are defined in the specification. A pharmacodynamic test shows that the compound can be used for treating diseases with the histopathologic characteristic of an IDO1-mediated tryptophan metabolism pathway, such as malignant tumors, autoimmune diseases, Alzheimer's disease and schizophrenia. (The formula is shown in the specification).
Owner:CHINA PHARM UNIV
Benzonitrogen-containing heterocyclic indoleamine-2,3-dioxygenase 1 inhibitors and uses thereof
ActiveCN106883224BStrong inhibitory activityOrganic active ingredientsNervous disorderDiseaseBenzene
The invention relates to the field of pharmaceutical chemistry and particularly relates to an iodoleamine-2,3-dioxygenase 1 inhibitor (I), represented by a formula (I) shown in the description, with a nitrogen-containing benzoheterocyclic structure, wherein X, R, n and m are as defined in the description. Proven by pharmacodynamic tests, the compound disclosed by the invention can be used for treating diseases with IDO1 mediated pathological features of a tryptophan metabolism pathway such as malignant tumors, autoimmune diseases, Alzheimer's disease and schizophrenia.
Owner:CHINA PHARM UNIV
Application of albiflorin as indoleamine 2,3-dioxygenase (IDO) inhibitor
InactiveCN107496434AGood curative effectSafe and effective treatmentOrganic active ingredientsSenses disorderFood additiveDisease
The invention provides an application of albiflorin, or a pharmaceutically acceptable salt thereof, as an IDO inhibitor. The albiflorin or the pharmaceutically acceptable salt thereof herein is used as the IDO inhibitor for preparing medicines, foods, healthcare products, food additives or nutritious supplements, which are used for preventing and treating pathological feature diseases with tryptophan metabolic pathway, which are induced by IDO over-activation; herein, the diseases are selected from one or more of cancer, depression, Alzheimer's disease and Parkinson's disease.
Owner:张作光
Nitrogen-containing benzoheterocyclic iodoleamine-2,3-dioxygenase 1 inhibitor and use thereof
ActiveCN106883224AStrong inhibitory activityOrganic active ingredientsNervous disorderDiseaseBenzene
The invention relates to the field of pharmaceutical chemistry and particularly relates to an iodoleamine-2,3-dioxygenase 1 inhibitor (I), represented by a formula (I) shown in the description, with a nitrogen-containing benzoheterocyclic structure, wherein X, R, n and m are as defined in the description. Proven by pharmacodynamic tests, the compound disclosed by the invention can be used for treating diseases with IDO1 mediated pathological features of a tryptophan metabolism pathway such as malignant tumors, autoimmune diseases, Alzheimer's disease and schizophrenia.
Owner:CHINA PHARM UNIV
Phthalimide IDO1 (indoleamine-2,3-dioxygenase 1) inhibitor and use thereof
The invention relates to the field of medicinal chemistry, in particular to an IDO1 (indoleamine-2,3-dioxygenase 1) inhibitor (I) of a phthalimide structure and a preparation method, wherein X and R are defined in the specification. A pharmacodynamic test shows that the compound can be used for treating diseases with the histopathologic characteristic of an IDO1-mediated tryptophan metabolism pathway, such as malignant tumors, autoimmune diseases, Alzheimer's disease and schizophrenia. (The formula is shown in the specification).
Owner:CHINA PHARM UNIV
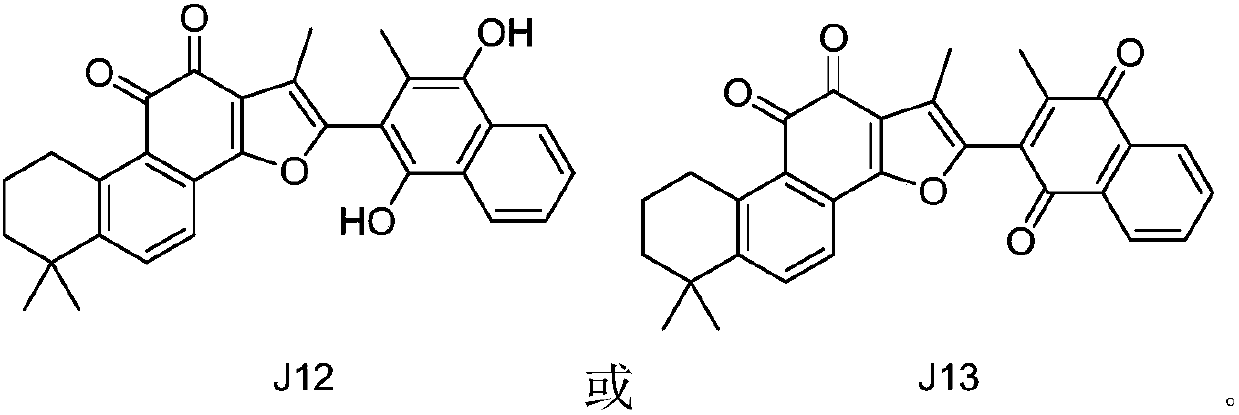
Indoleamine 2, 3-dioxygenase inhibitor containing tanshinone compound
The invention relates to the technical field of indoleamine 2, 3-dioxygenase inhibitors, in particular to an indoleamine 2, 3-dioxygenase inhibitor with a tanshinone compound as the active substance.IDO inhibitory activity detection shows that the tanshinone compound of the structure or pharmaceutically acceptable salts thereof can have an inhibitory effect on IDO, and can obstruct and / or damagethe promotion effect of IDO in occurrence and development of diseases, thus providing good prospects for treatment of IDO mediated tryptophan metabolic pathway pathological feature diseases.
Owner:SUN YAT SEN UNIV
N-alkyl tryptanthrin derivative, preparation method for same, and application thereof
ActiveUS20160168152A1Novel structureHighly effectiveOrganic active ingredientsSenses disorderDiseaseTryptophan
An N-alkyl tryptanthrin derivative, a preparation method for same, and an application thereof are provided. The structure of the derivative is as represented by formula I. The N-alkyl tryptanthrin derivative can serve as a highly active IDO inhibitor, for use in preparing a medicament for prevention and / or treatment of a disease having a pathological characteristic of an IDO-mediated tryptophan metabolic pathway.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Application of coptisine serving as indoleamine 2, 3-dioxygenase (IDO)inhibitor
InactiveCN102579438AExcellent inhibitory effectConvenient researchOrganic active ingredientsNervous disorderDiseaseTryptophan
The invention belongs to the field of medicine and particularly relates to an application of coptisine serving as an indoleamine 2, 3-dioxygenase (IDO) inhibitor. The coptisine is an IDO reversible inhibitor, the inhibitor constant Ki is 5.76mu M, the half effective inhibition concentration (IC50) in vitro and at a cell level is 6.31mu M and 7.11mu M respectively, and the inhibition effect is obviously better than that of existing inhibitor of 1-methyl trytophan. According to the application of the coptisine serving as the indoleamine 2, 3-dioxygenase inhibitor, the coptisine is used as the IDO inhibitor so that IDO activity which rises abnormally in a neuron cell model and an animal model in alzheimer disease is effectively lowered, functions of curing alzheimer disease are achieved, and the functions include a protective function to neuron cells, an anti-apoptosis function and a function of improving learning and memory capabilities of animals having alzheimer disease. The coptisine and hydrochloride of the coptisine are used as an IDO inhibitor so that the application prospect is wide, and the coptisine and the hydrochloride of the coptisine can be used for curing serious diseases with pathological characteristics of IDO mediates and trytophan metabolic pathways such as alzheimer disease, depressive disorders, cancers, acquired immune deficiency syndrome, cataract and the like.
Owner:FUDAN UNIV
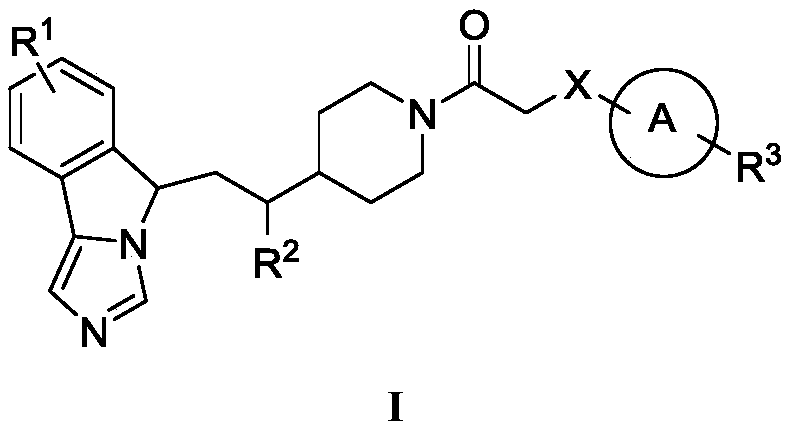
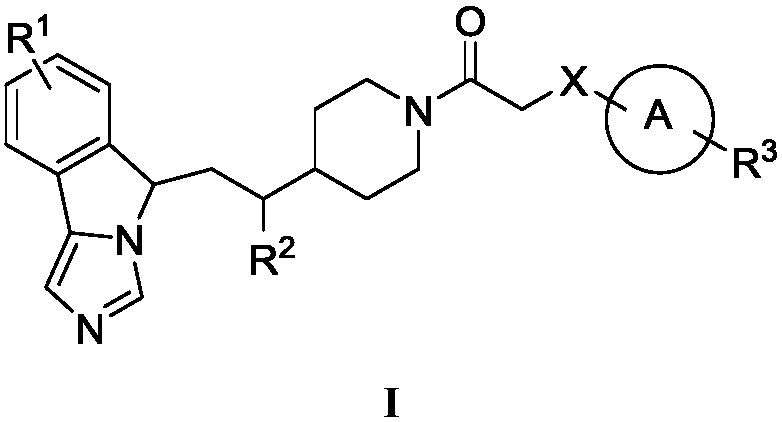
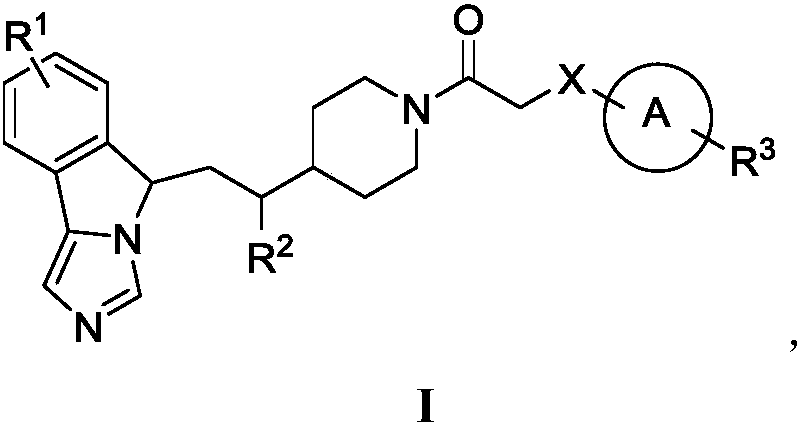
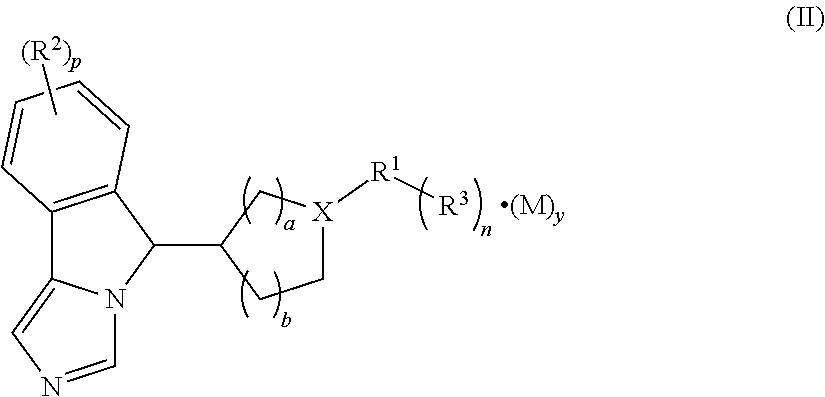
Imidazo isoindole derivative, preparation method therefor and medical use thereof
ActiveUS20180118750A1Significant effectLess side effectsOrganic active ingredientsNervous disorderAutoimmune conditionAutoimmune disease
The present invention relates to an imidazo isoindole derivative, a preparation method therefor and a medical use thereof. In particular, the present invention relates to the imidazo isoindole derivative as shown in the formula (I), a preparation method and pharmaceutical composition containing the derivative, and a use thereof for treating diseases with a pathological characteristic of the IDO-mediated tryptophan metabolic pathways. The diseases comprise cancers, Alzheimer's disease, autoimmune diseases, depression, anxiety disorders, cataracts, psychological disorders and AIDS, wherein the substituents in the formula (I) are the same as those defined in the specification.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Application of quassia alkaloids serving as indoleamine-2,3-dioxygenase (IDO) inhibitor
The invention provides application of natural quassia alkaloids or pharmacologically acceptable salts of the natural quassia alkaloids serving as an indoleamine-2,3-dioxygenase (IDO) inhibitor. The quassia alkaloids or the pharmacologically acceptable salts of the quassia alkaloids serving as the IDO inhibitor can be used for preparing drugs, food, health products, food additives or nutritional supplements for preventing and / or curing pathological feature diseases caused by the fact that tryptophan metabolic pathways are mediated by IDO over-activation, and the diseases are selected from one or more of cancers, depression, Alzheimer disease and Parkinsonism.
Owner:YANTAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com