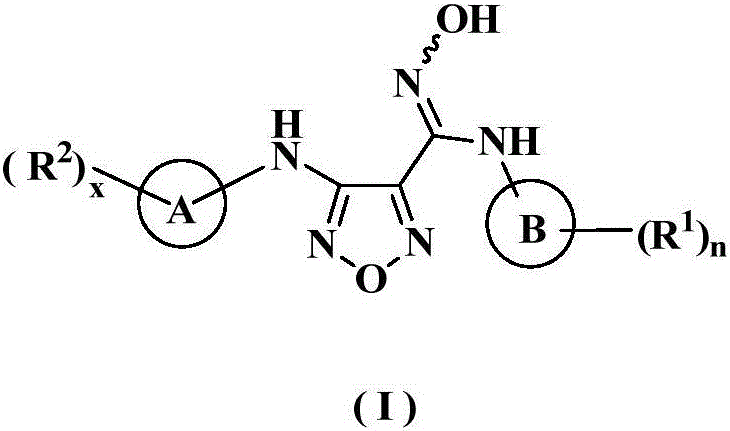
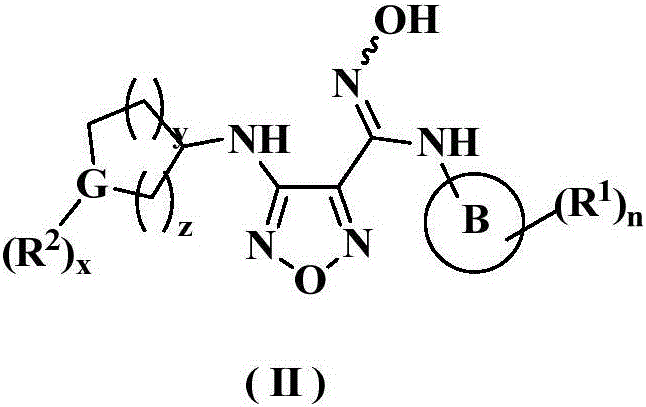
Oxadiazole derivative, preparation method therefor and use of oxadiazole derivative in medicines
A technology for compounds and mixtures, applied to oxadiazole derivatives, their preparation and their application in medicine, can solve the problems of not finding good IDO inhibitors and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
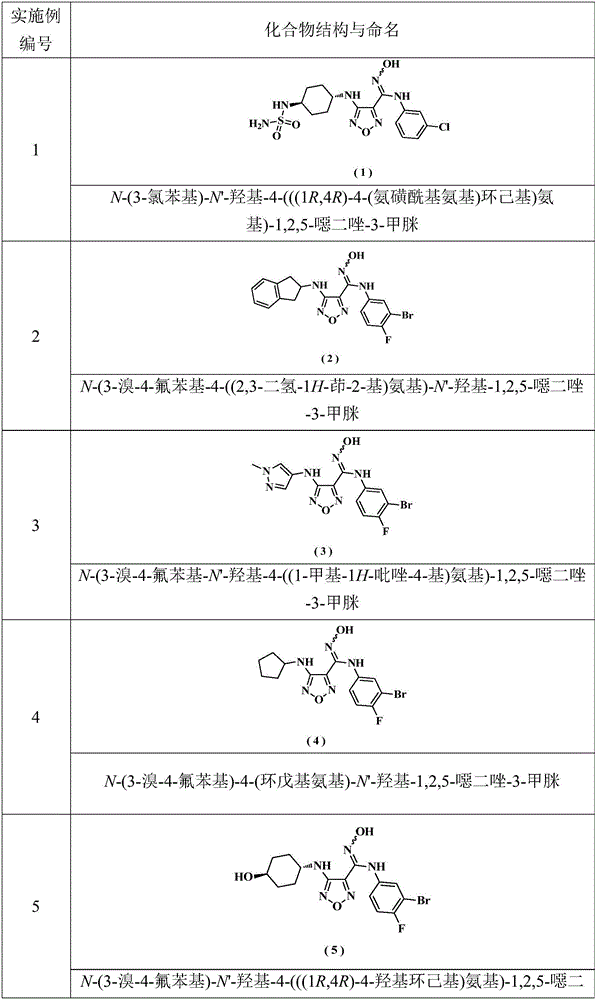
Embodiment 1
[0162] N-(3-chlorophenyl)-N'-hydroxy-4-(((1R,4R)-4-(sulfamoylamino)cyclohexyl)amino)-1,2,5-oxadiazole-3 - formamidine
[0163]
[0164] first step
[0165] 4-(3-Chlorophenyl)-3-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5(4H)-one 1b
[0166] 3-(4-Amino-1,2,5-oxadiazol-3-yl)-4-(3-chlorophenyl)-1,2,4-oxadiazol-5(4H)-one 1a (2.0g, 7.15mmol, prepared by the method disclosed in the patent application "WO2006122150") was dissolved in 5mL of trifluoroacetic acid, added 5mL of 30% hydrogen peroxide solution, heated to 45°C and stirred for 12 hours. The reaction solution was cooled to 25°C, diluted with 20 mL of ethyl acetate, quenched with 20 mL of saturated sodium thiosulfate solution, separated, the aqueous phase was extracted with ethyl acetate (15 mL×2), the organic phases were combined, anhydrous sodium sulfate After drying and filtering, the filtrate was concentrated under reduced pressure, and the residue was purified by high performance liquid chromatography to obtai...
Embodiment 2
[0185] N-(3-bromo-4-fluorophenyl-4-((2,3-dihydro-1H-inden-2-yl)amino)-N'-hydroxyl-1,2,5-oxadiazole- 3-Formamidine
[0186]
[0187] first step
[0188] 4-(3-Bromo-4-fluorophenyl)-3-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazole-5(4H )-one 2b
[0189] 3-(4-amino-1,2,5-oxadiazol-3-yl)-4-(3-bromo-4-fluorophenyl)-1,2,4-oxadiazol-5(4H )-ketone 2a (13.0 g, 41.1 mmol, prepared by the method disclosed in patent application "WO2014066834") was added to 150 mL of trifluoroacetic acid, 90 mL of aqueous hydrogen peroxide (30%), and reacted at 45°C for 48 hours. After the reaction, cool down, add 300mL saturated sodium thiosulfate solution and 150mL ethyl acetate, stir and react for 20 minutes, and detect no peroxide with potassium iodide test paper. The liquid was separated, the aqueous phase was extracted with ethyl acetate (100 mL×2), the organic phases were combined, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the obt...
Embodiment 3
[0198] N-(3-Bromo-4-fluorophenyl-N'-hydroxy-4-((1-methyl-1H-pyrazol-4-yl)amino)-1,2,5-oxadiazole-3 - formamidine
[0199]
[0200] 4-(3-bromo-4-fluorophenyl)-3-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5( 4H)-ketone 2b (100mg, 0.27mmol), 1-methyl-1H-pyrazol-4-amine 3a (26.1mg, 0.27mmol) was dissolved in 5mL tetrahydrofuran, and 32.4mL 2.5M sodium hydroxide solution was added, The reaction was stirred for 1.5 hours. After the reaction, pour the reaction solution into ice water, adjust the pH to neutral with 1N hydrochloric acid, extract with ethyl acetate, dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and use thin-layer chromatography to eluent system The resulting residue was purified by A to afford the title product 3 (10 mg, yellow solid) in 10% yield.
[0201] MS m / z(ESI):396.3 / 398.3[M+1]
[0202] 1 H NMR (400MHz, DMSO-d 6 )δ11.52(s,1H),9.02(s,1H),8.61-8.62(m,1H),7.79-7.80(m,1H),7.46-7.48(m,1H),7.17-7.19(m, 1H),6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com