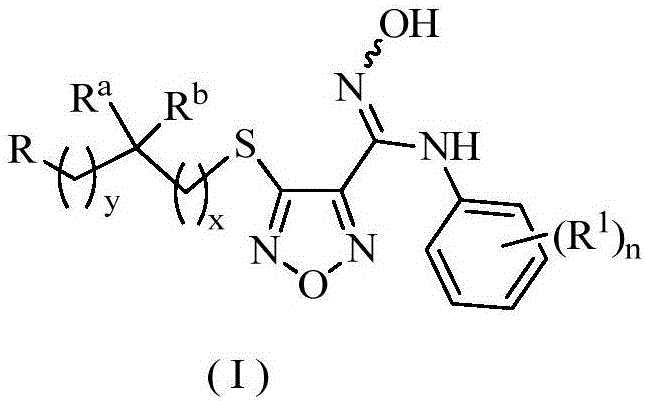
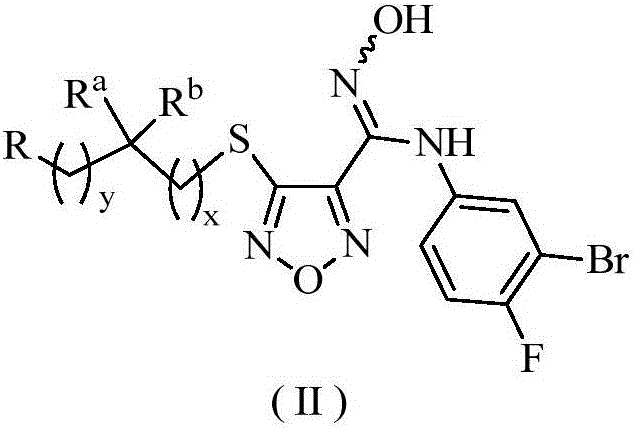
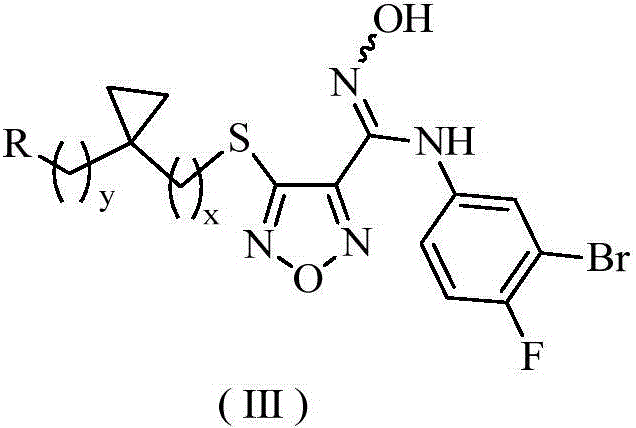
Oxadiazole derivative, preparing method of oxadiazole derivative and application of oxadiazole derivative to medicines
A technology of compounds and mixtures, applied in the field of oxadiazole derivatives, their preparation and their application in medicine, can solve the problems of not finding a good IDO inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] (S)-N-(3-bromo-4-fluorophenyl)-4-((2,3-dihydroxypropyl)thio)-N'-hydroxy-1,2,5-oxadiazole- 3-Formamidine
[0172]
[0173]
[0174] first step
[0175] 4-(3-Bromo-4-fluorophenyl)-3-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazole-5(4H )-ketone
[0176] 3-(4-amino-1,2,5-oxadiazol-3-yl)-4-(3-bromo-4-fluorophenyl)-1,2,4-oxadiazol-5(4H )-ketone 1a (13.0 g, 41.1 mmol, prepared by the method disclosed in patent application "WO2014066834") was added to 150 mL of trifluoroacetic acid, 90 mL of hydrogen peroxide solution (30%), and reacted at 45°C for 48 hours. After the reaction, cool down, add 300mL saturated sodium thiosulfate solution and 150mL ethyl acetate, stir and react for 20 minutes, and detect no peroxide with potassium iodide test paper. Separate the liquids, extract the aqueous phase with ethyl acetate (100 mL×2), combine the organic phases, dry over anhydrous sodium sulfate, filter, concentrate the filtrate under reduced pressure, and purify the resulting r...
Embodiment 2
[0190] (R)-N-(3-bromo-4-fluorophenyl)-4-((2,3-dihydroxypropyl)thio)-N'-hydroxy-1,2,5-oxadiazole- 3-Formamidine
[0191]
[0192] first step
[0193] (R)-4-(3-bromo-4-fluorophenyl)-3-(4-(((2,2-dimethyl-1,3-dioxolan-4-yl)methyl) Thio)-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5(4H)-one
[0194] 4-(3-bromo-4-fluorophenyl)-3-(4-nitro-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5( 4H)-Kone 1b (300 mg, 0.81 mmol) was dissolved in 15 mL of tetrahydrofuran, and (R)-(2,2-dimethyl-1,3-dioxolan-4-yl)methanol 2a (143 mg , 0.96mmol, prepared by the known method "European Journal of Organic Chemistry, 2006, (21), 4805-4812"), potassium carbonate (223mg, 1.62mmol) was added, and reacted at 25°C for 48 hours. After the reaction, the title product (R)-4-(3-bromo-4-fluorophenyl)-3-(4-(((2,2-dimethyl-1,3-dioxolane- 4-yl)methyl)thio)-1,2,5-oxadiazol-3-yl)-1,2,4-oxadiazol-5(4H)-one 2b, without purification Proceed directly to the next reaction.
[0195] second step
[0196] (R)-N-(3-bromo-4-fluor...
Embodiment 3
[0204] N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((2-(1-(sulfamoylamino)cyclopropyl)ethyl)thio)-1,2,5 -Oxadiazole-3-carboxamidine
[0205]
[0206] first step
[0207] S-(2-(1-((tert-butoxycarbonyl)amino)cyclopropyl)ethyl)acetylsulfate
[0208] Dissolve 2-(1-((tert-butoxycarbonyl)amino)cyclopropyl)ethylmethylsulfonate 3a (1.2g, 4.3mmol, prepared by the method disclosed in the patent application "WO2013020993") in 15mL Add thioacetic acid (327 mg, 4.3 mmol) and cesium carbonate (1.39 g, 4.3 mmol) to tetrahydrofuran, and react at 65° C. for 1 hour. After the reaction, the reaction solution was concentrated under reduced pressure, 50 mL of water was added, extracted with ethyl acetate (50 mL×3), the organic phases were combined, dried with anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain the crude title product S- (2-(1-((tert-butoxycarbonyl)amino)cyclopropyl)ethyl)acetylsulfate 3b (200 mg, yellow oil), the product was dir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com