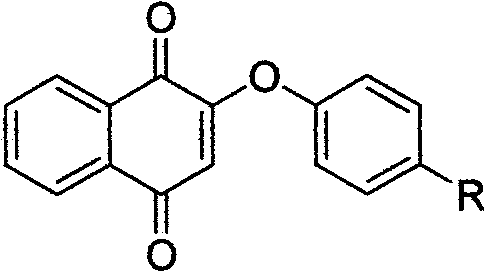
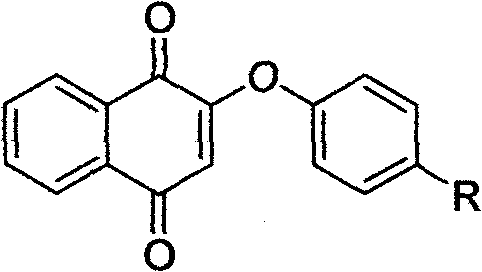
Vitamin K3 derivatives serving as IDO inhibitor
A technology of derivatives and vitamins, which is applied in the field of synthesis of drugs and intermediates, can solve the problems of lack of high yield and achieve the effects of solvent saving, mild reaction conditions and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
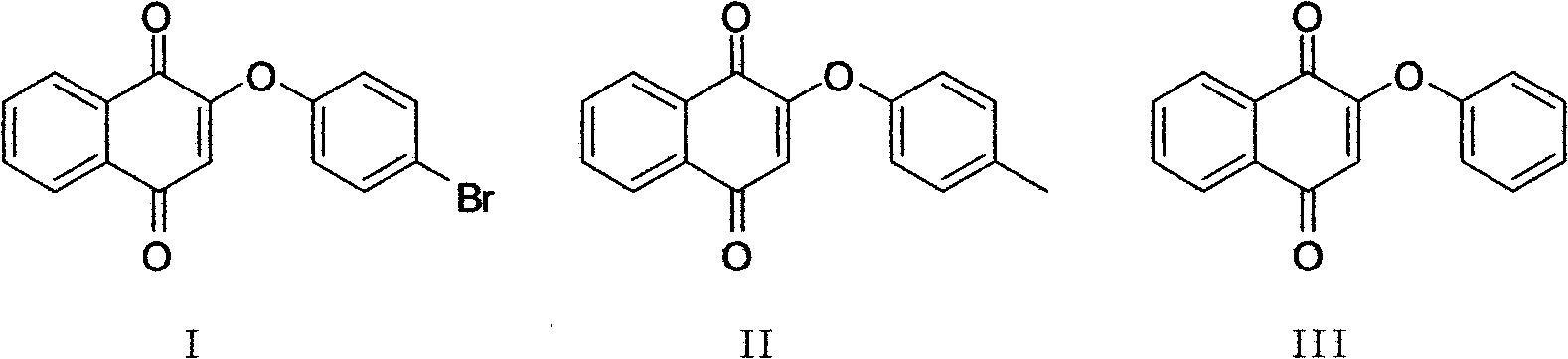
[0036] Embodiment 1: the preparation of compound I
[0037]
[0038] Add 4mL dimethylformamide (DMF) to a 50mL dry round-bottomed flask, add 18mg (0.1mmol) of p-bromophenol and 42mg (0.3mmol) of anhydrous potassium carbonate respectively, track and monitor the generation of potassium salt by TLC, magnetic After stirring for 1 hour, after it generates potassium phenate; 24mg (0.1mmol) 2-bromo-1,4-naphthoquinone was poured into the system, and the system appeared brown. After reacting at room temperature for 3 hours, TLC tracked and monitored the progress of the reaction. Completely quench the reaction with 20 mL of water, add ethyl acetate, separate the organic layer, add saturated sodium bisulfite, and wash with saturated brine, dry with anhydrous sodium sulfate, and use ethyl acetate:petroleum ether=1 : 8 is the eluent and is separated and purified by column chromatography to obtain 40 mg of compound I with a yield of 75%.
[0039] The characterization data are as follows...
Embodiment 2
[0041] Embodiment 2: the preparation of compound II
[0042]
[0043]Add 4mL DMF to the dry round-bottomed flask of 50mL, add the anhydrous potassium carbonate of 16mg (0.15mmol) p-cresol and 62mg (0.45mmol) respectively, TLC follows and monitors potassium salt to generate, after magnetic stirring 2.5 hours, wait for its After the potassium phenate is generated; pour 24mg (0.1mmol) 2-bromo-1,4-naphthoquinone into the system, and the system turns brown. After reacting at room temperature for 4 hours, TLC tracked and monitored the reaction to complete, and quenched it with 20mL of water For post-reaction treatment, add ethyl acetate, separate the organic layer, then add saturated sodium bisulfite and saturated brine to wash, dry over anhydrous sodium sulfate, and use ethyl acetate:petroleum ether=1:8 as the eluent for column After separation and purification by chromatography, 30 mg of compound II was obtained, with a yield of 70%.
[0044] The characterization data are as f...
Embodiment 3
[0046] Embodiment 3: the preparation of compound III
[0047]
[0048] Add 4mL DMF to a 50mL dry round-bottom flask, add 14mg (0.15mmol) of phenol and 62mg (0.45mmol) of anhydrous potassium carbonate respectively, track and monitor the generation of potassium salt by TLC, and wait for it to generate phenol after 1 hour of magnetic stirring. After the potassium salt; 24mg (0.1mmol) 2-bromo-1,4-naphthoquinone was poured into the system, and the system showed a reddish-brown color. After 1.5 hours of reaction at room temperature, the TLC tracking monitoring reaction was completed, and the reaction was quenched with 20mL of water. After treatment, add ethyl acetate, separate the organic layer, then add saturated sodium bisulfite and saturated brine to wash, dry over anhydrous sodium sulfate, and use ethyl acetate:petroleum ether=1:7 as eluent for column layer 24 mg of compound III was obtained through separation and purification, with a yield of 81%.
[0049] The characterizat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com