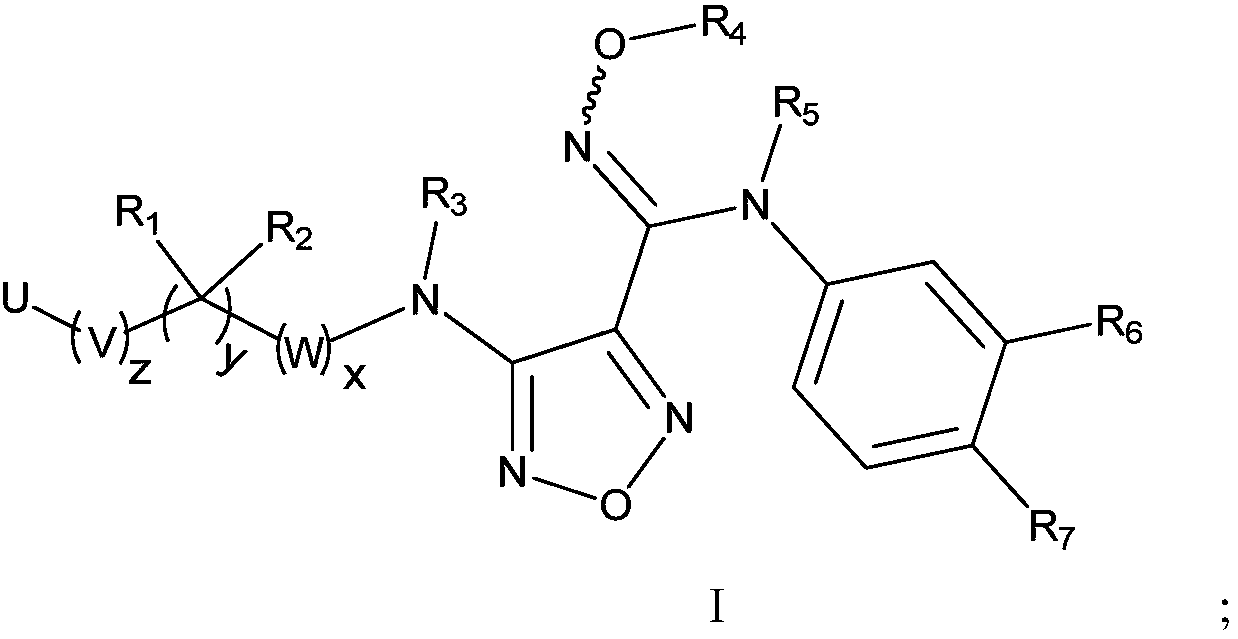
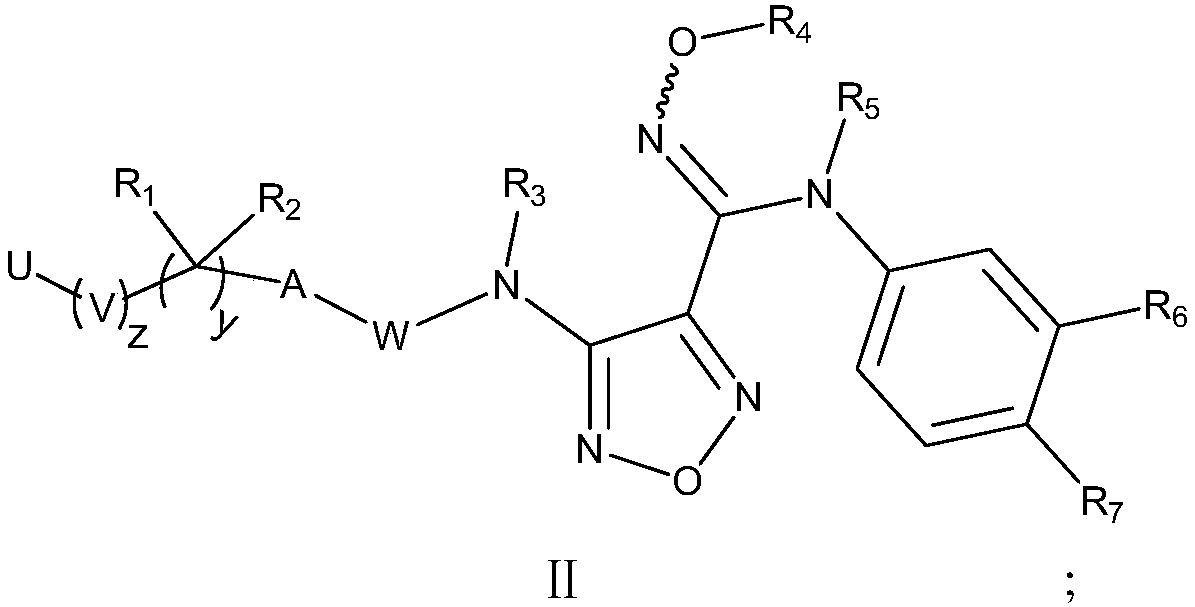
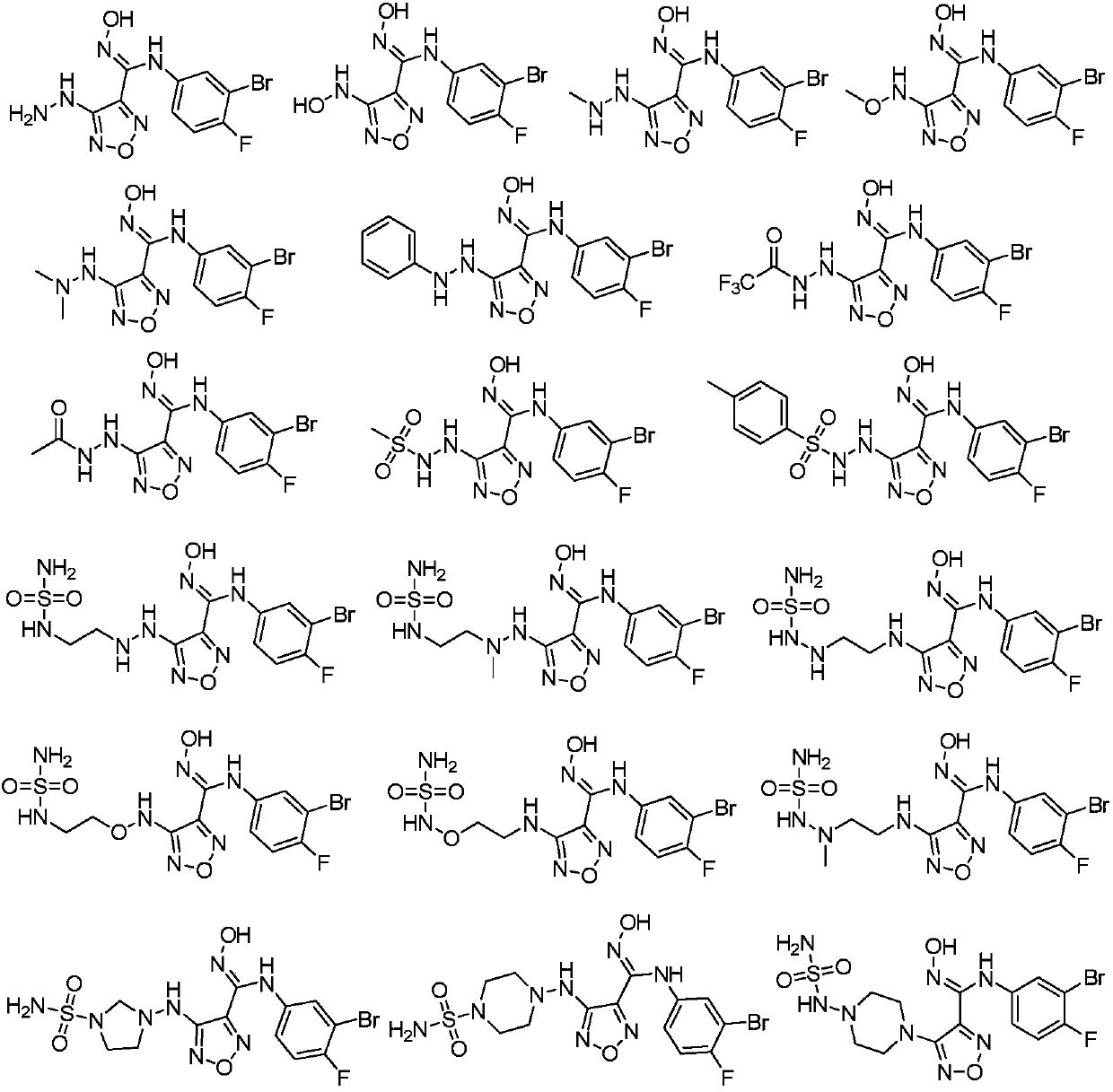
Compound used as IDO (Indoleamine-2,3-dioxygenase) regulator and application thereof
A compound and mixture technology, applied in the field of medicine, can solve problems such as no clinical development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0267]
[0268] The synthesis steps are as follows:
[0269] Synthesis of Intermediate 3-1:
[0270] Intermediate 1-1 (2g) was dissolved in tetrahydrofuran, then 80% hydrazine hydrate (1mL) was added, and then the reaction was reacted at room temperature for 2 hours, TLC showed that the raw material was completely reacted, and then 100ml of hydrazine hydrate was added to the reaction system Ethyl acetate was washed with saturated brine, the organic phase was dried and spin-dried, and passed through a column to obtain intermediate 3-1 (1.1 g, 57%). MS(M+H) + :m / z=356.9, 358.9; 1 HNMR (400MHz, CDCl 3 ): 7.64-7.62 (m, 1H), 7.34-7.27 (m, 2H), 6.54 (br, 1H), 4.02 (br, 2H).
[0271] The synthesis of embodiment 1:
[0272] Dissolve intermediate 3-1 (200 mg) in tetrahydrofuran, then add 4 equivalents of hydrazine hydrate at 0°C, and then slowly raise the temperature to room temperature for reaction. After TLC shows that intermediate 3-1 disappears, adjust the pH of the reactio...
Embodiment 12
[0278]
[0279] The synthesis steps of the target compound Example 12 are as follows.
[0280] Synthesis of compound 15:
[0281] Dissolve chlorosulfonic acid isocyanate (compound 14, 14.1g, 0.1mol) in 100ml of dry dichloromethane, then add tert-butanol (7.4g, 0.2mol) in dichloromethane dropwise under the protection of argon under ice-water bath cooling (10ml) solution, stirred in an ice-water bath for 30min to form solution A. Triethylamine (20.2 g, 0.2 mol) was added dropwise to a suspension of glycine ethyl ester hydrochloride (14 g, 0.1 mol) in DCM (200 ml) under cooling in an ice-water bath, followed by stirring for 15 min to form solution B. Under an ice-water bath, solution A was added dropwise to solution B, then naturally warmed to room temperature and stirred overnight. The reaction solution was diluted with 100ml of dichloromethane, then added with 100ml of saturated ammonium chloride solution and stirred for 5min, separated, the organic phase was dried over an...
Embodiment 13
[0292]
[0293] The synthesis steps of the target compound Example 13 are specifically referred to in Example 12, and the characterization information of the final product Example 13 is as follows: 1 HNMR(MeOD,400MHz)δ=7.22-7.20(q,1H),7.12-7.08(t,1H),6.93-6.89(m,1H),3.68(s,2H),3.21(s,3H).MS [M+H]481.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com