Compound capable of inhibiting IDO (indoleamine 2, 3-dioxyenase), and preparation method and application of compound
A technology for compounds and uses, applied in the field of medicine, can solve the problem of not finding a good IDO inhibitor, and achieve the effect of strong inhibitory activity and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
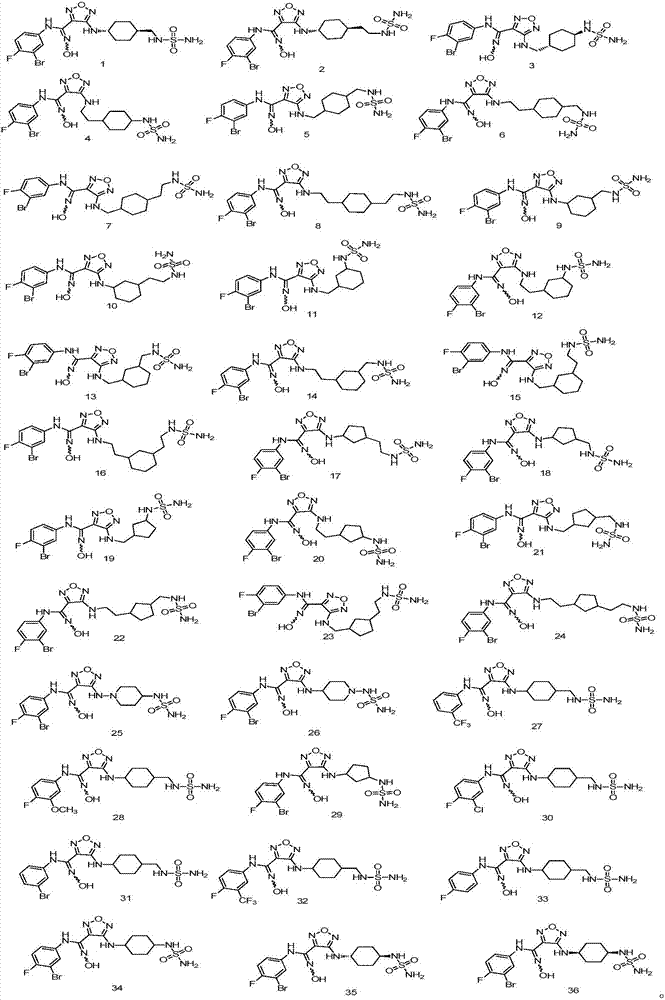
Examples
preparation example Construction
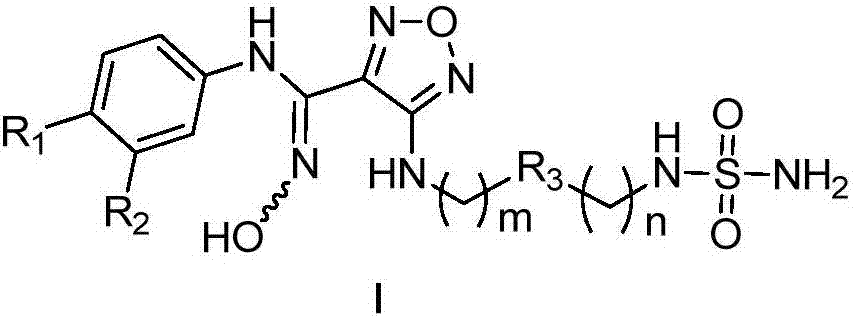
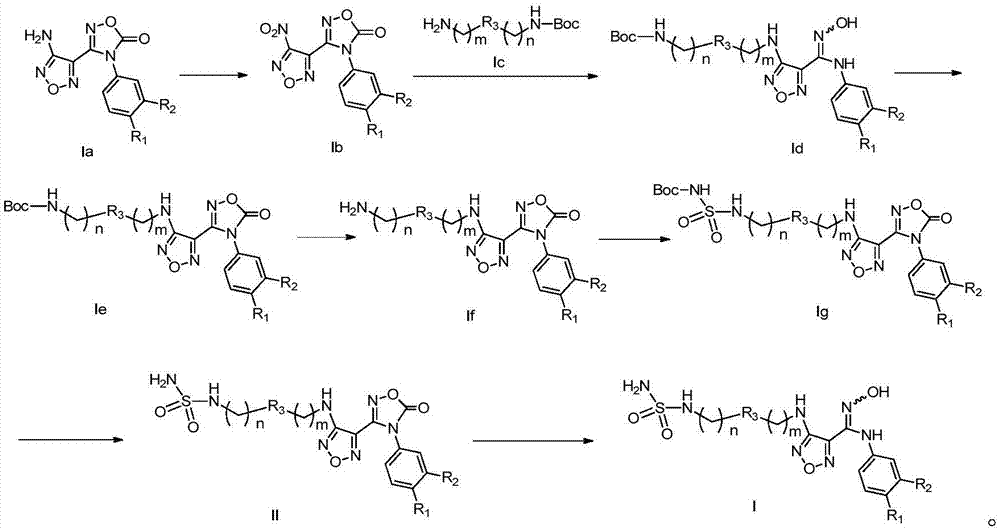
[0031] The method for preparing the compound represented by general formula (I) or its salt of the present invention includes the following steps:
[0032]
[0033] Under acidic conditions, compounds of general formula (Ia) are oxidized to compounds of general formula (Ib); compounds of general formula (Ib) react with compounds of general formula (Ic) under basic conditions to obtain compounds of general formula (Id); The compound of formula (Id) forms a ring under heating and basic conditions. The base under this condition is preferably N,N'-carbonyldiimidazole to obtain the compound of general formula (Ie); the compound of general formula (Ie) after ring formation is acidic Under conditions, the protective group on the amino group is removed to obtain the compound of general formula (If) or its salt; the basic solution of the compound of general formula (If) or its salt is reacted with the alcohol solution of chlorosulfonyl isocyanate at low temperature to obtain the general for...
Embodiment 1
[0046] trans-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((4-((sulfamoylamino)methyl)cyclohexyl)amino)-1,2,5-oxadiazole-3-carboximidamide, the compound The preparation of 1, wherein the structural formula of compound 1 is as follows:
[0047]
[0048] The first step is to prepare compound lb:
[0049] The raw material compound la (500 mg, 1.46 mmol, prepared by referring to the method disclosed in P53 Example3 step A in the patent application "WO2010005958") was added to 7 mL of trifluoroacetic acid, and then 6 mL of hydrogen peroxide (30%) was added, and reacted at 45°C for 20 hours . After the reaction, the reaction was quenched by adding saturated sodium sulfite solution, extracted with ethyl acetate, the organic phases were combined, dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue obtained was purified by silica gel column chromatography with eluent system B Compound lb (410 mg, yellow oil) was obtained with a y...
Embodiment 2
[0065] trans-N-(3-bromo-4-fluorophenyl)-N'-hydroxy-4-((4-(2-(sulfamoylamino)ethyl)cyclohexyl)amino)-1,2,5-oxadiazole-3-carboximidamide, That is, the preparation of compound 2, wherein the structural formula of compound 2 is as follows:
[0066]
[0067] Using the synthesis method of Example 1, the second step raw material compound 1c was replaced with (Commercially available), compound 2 (73 mg, white solid) was prepared with a yield of 45%. MS m / z(ESI): 521.4[M+1] + , 1 HNMR (400MHz, DMSO-d6) δ 11.52 (s, 1H), 8.87 (s, 1H), 7.09-7.27 (m, 3H), 6.76-6.79 (m, 1H), 6.67 (s, 2H), 6.21 (t,1H),2.41(m,1H),1.20-1.83(m,11H),3.55(d,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com