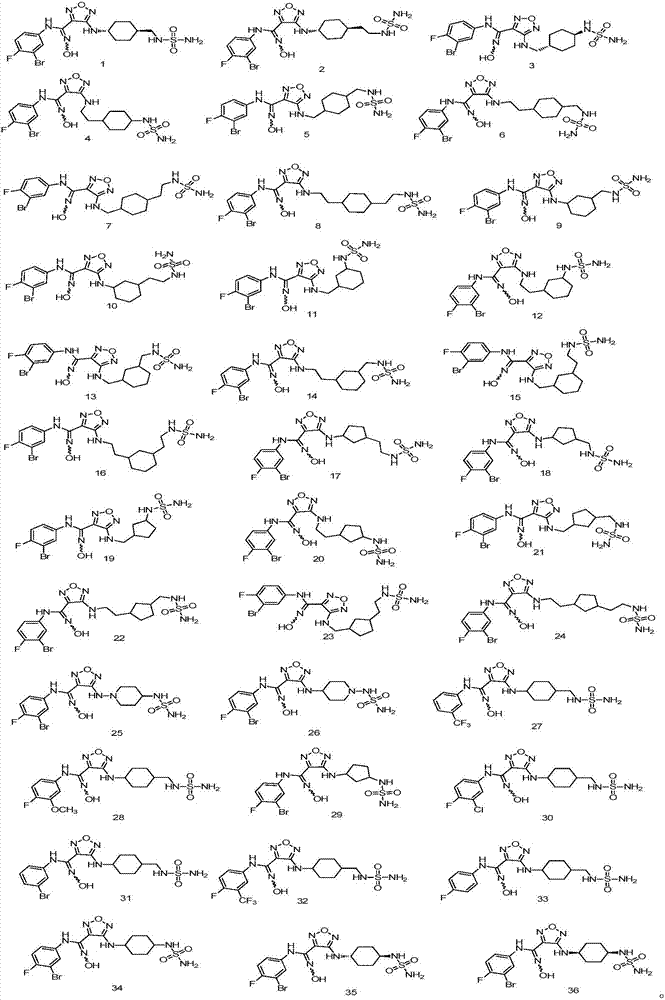
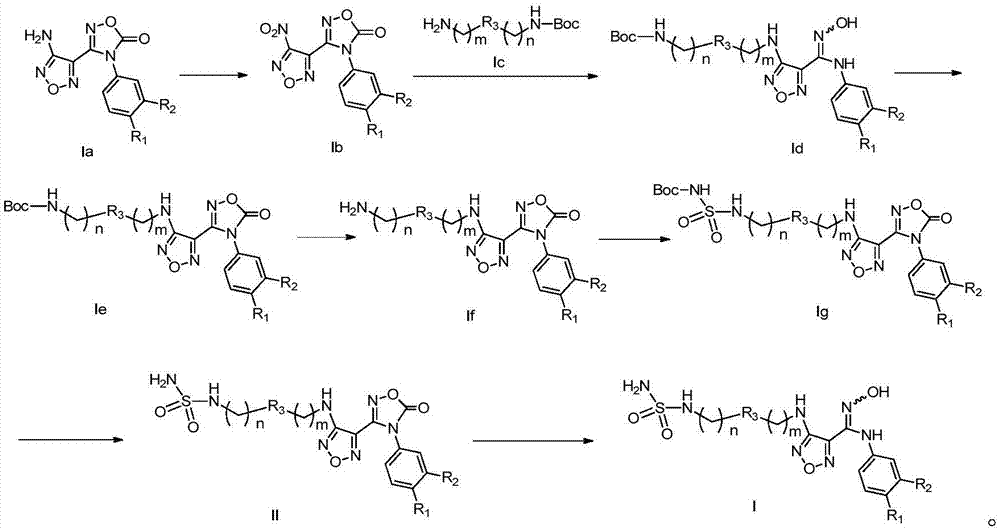
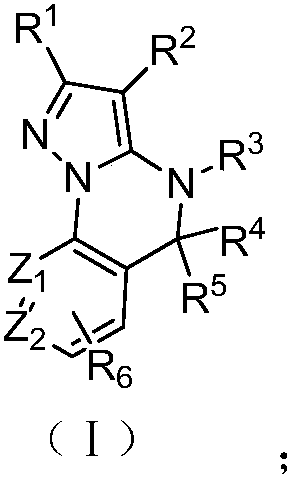
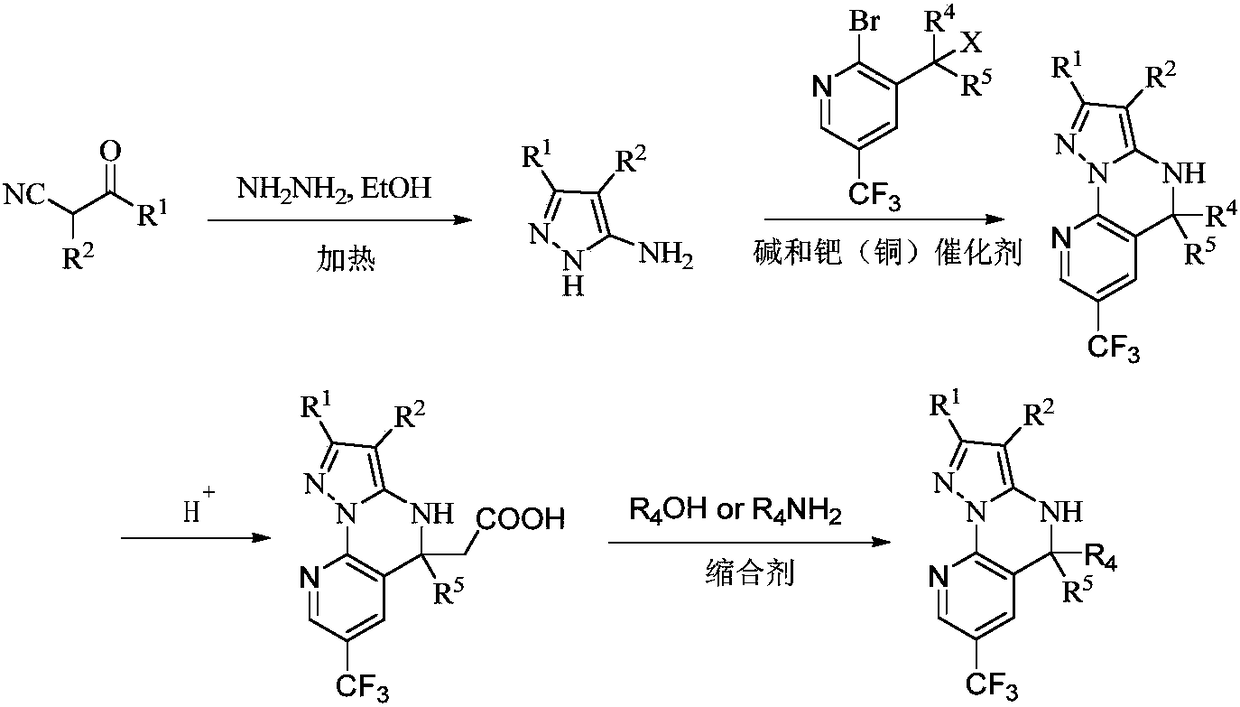
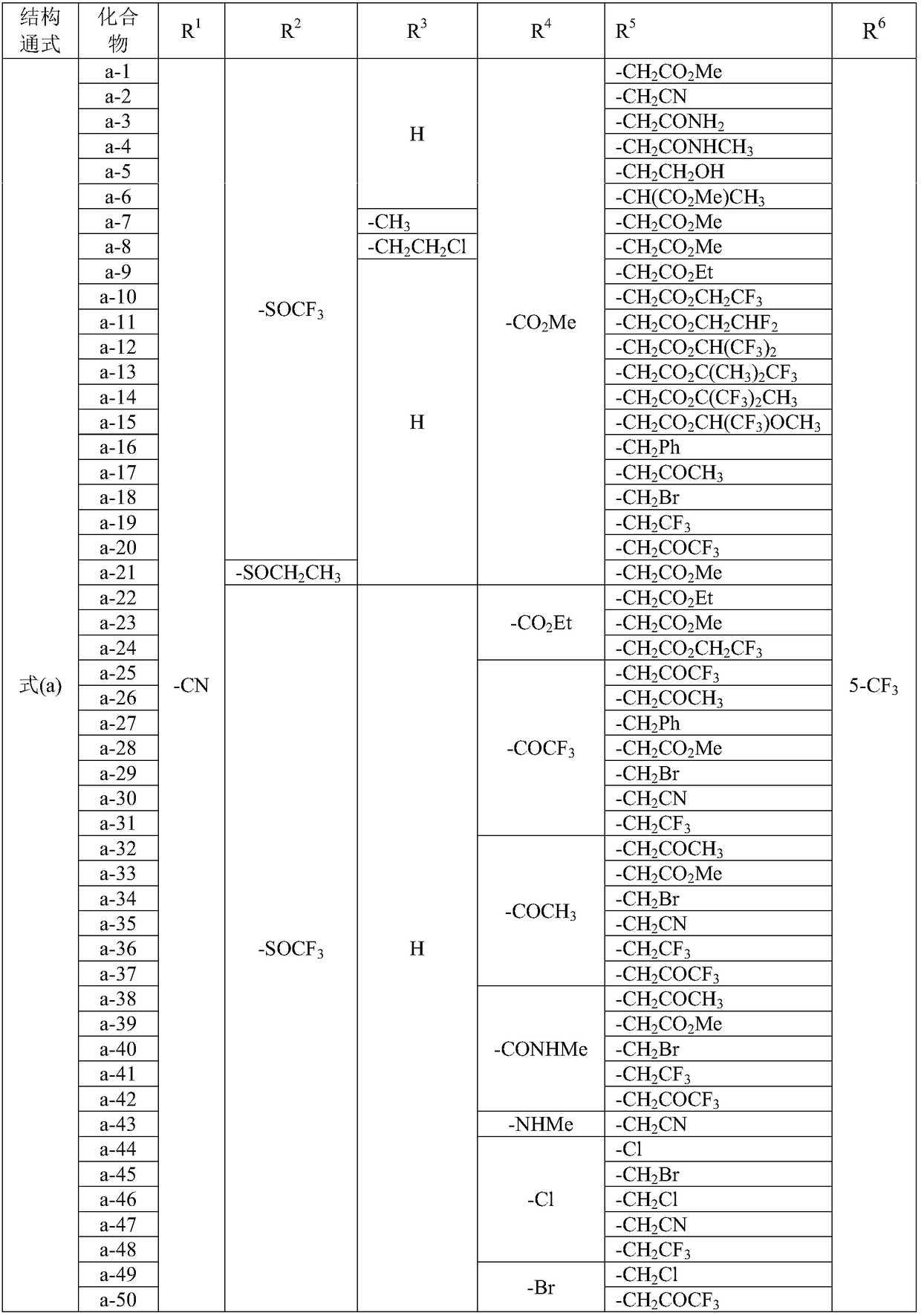
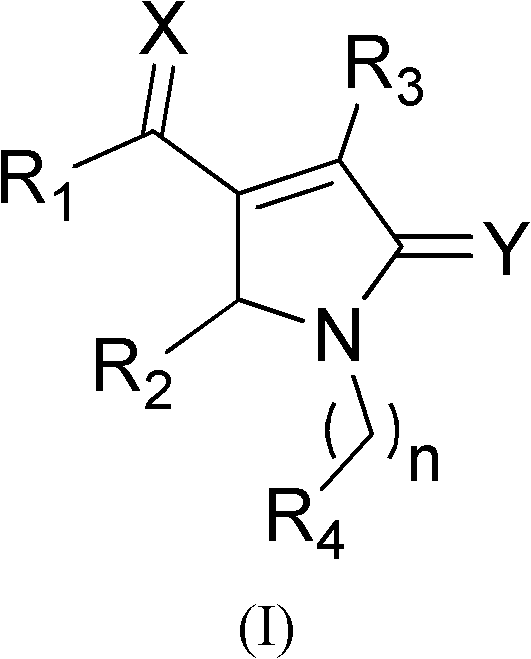
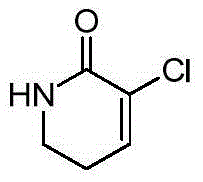
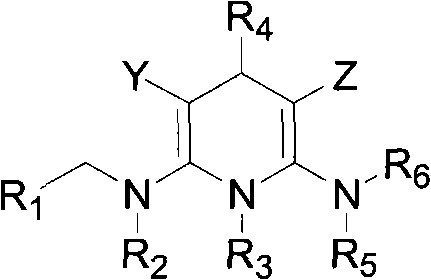
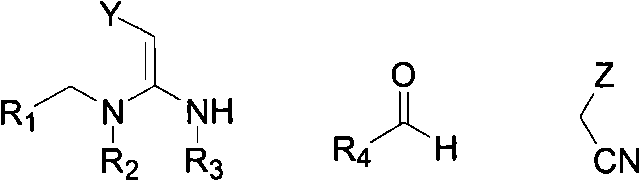
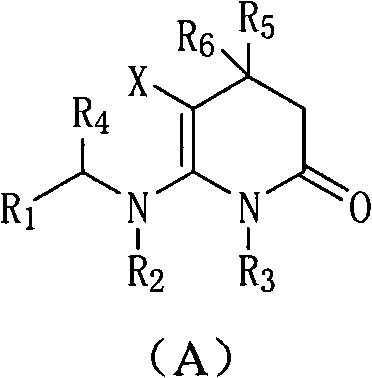
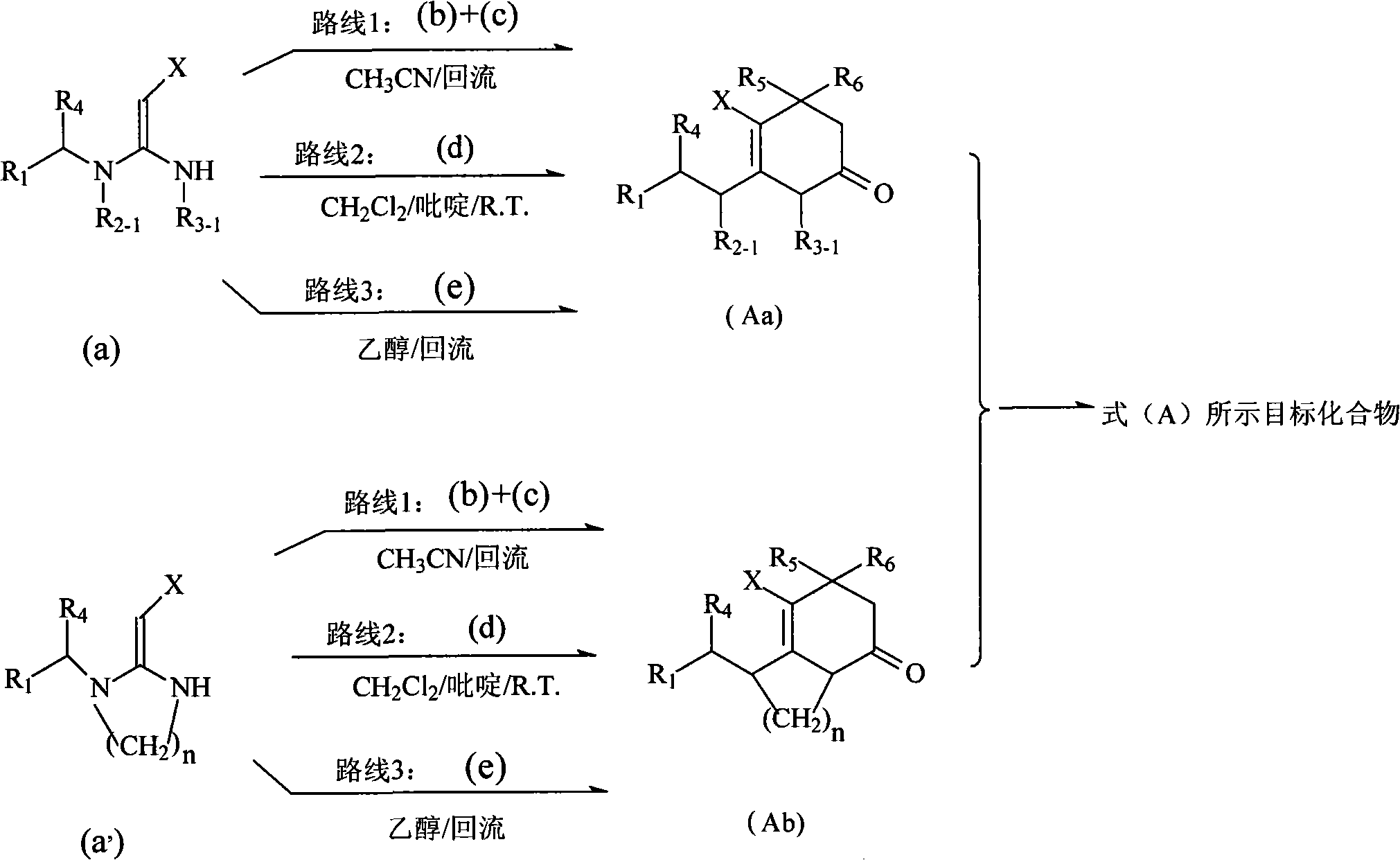
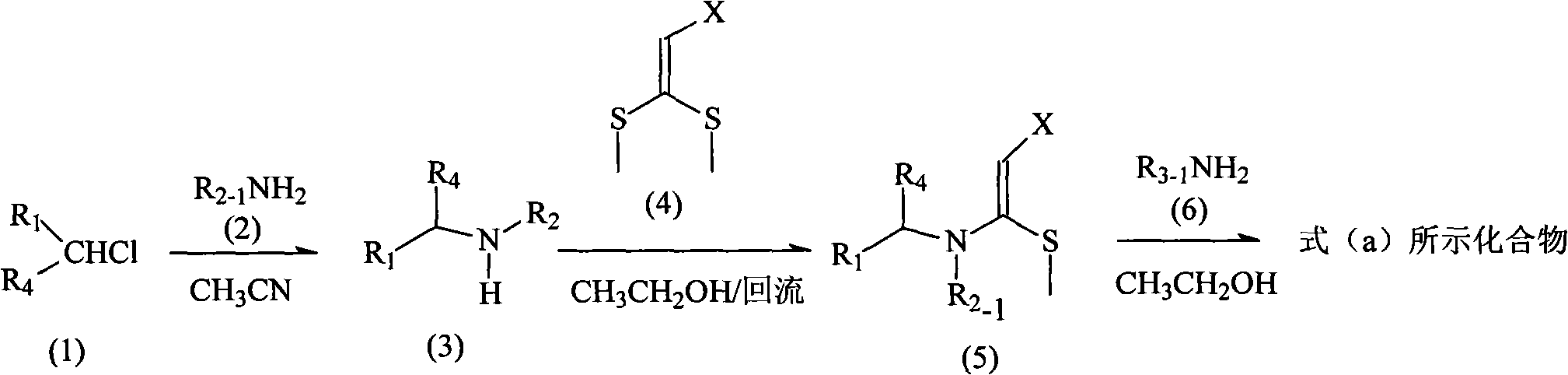
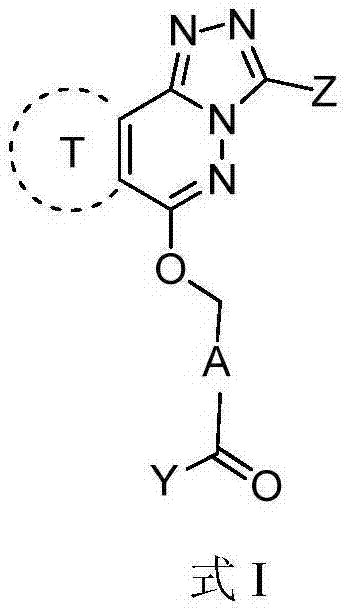
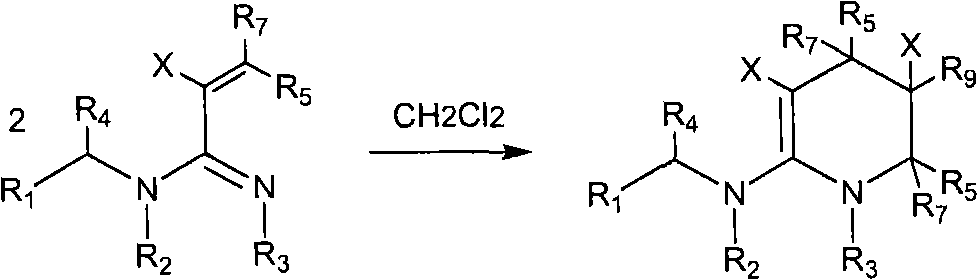
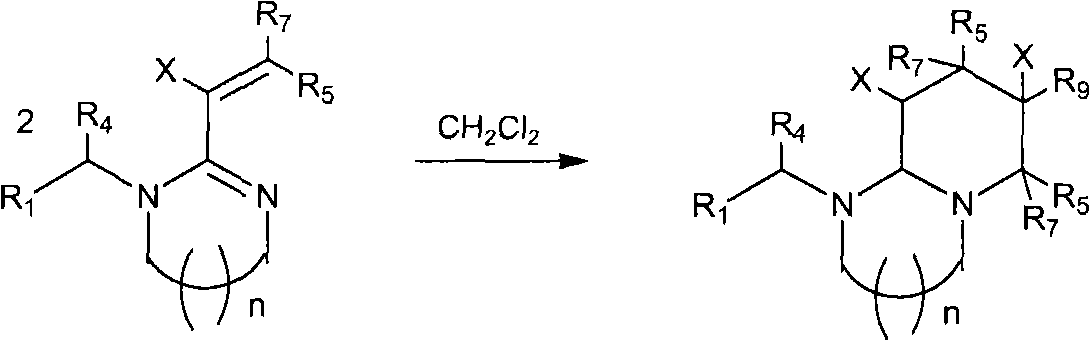
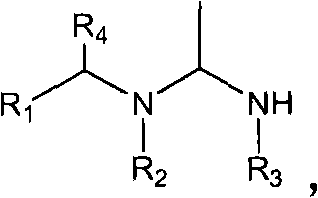
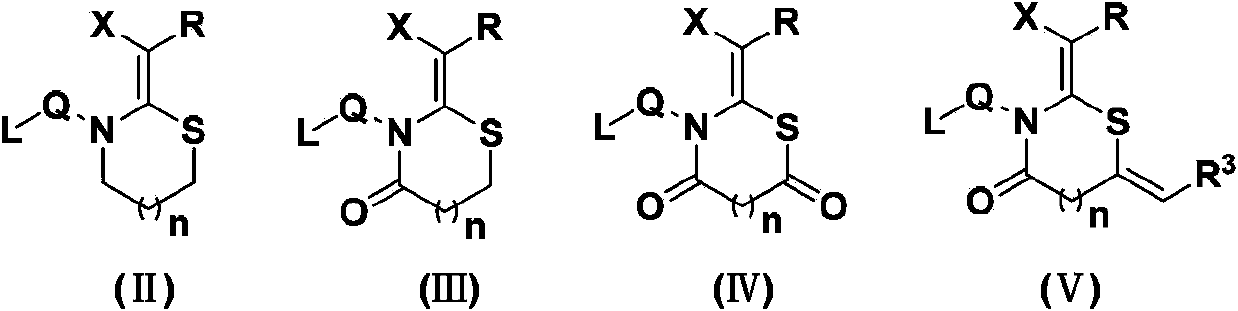
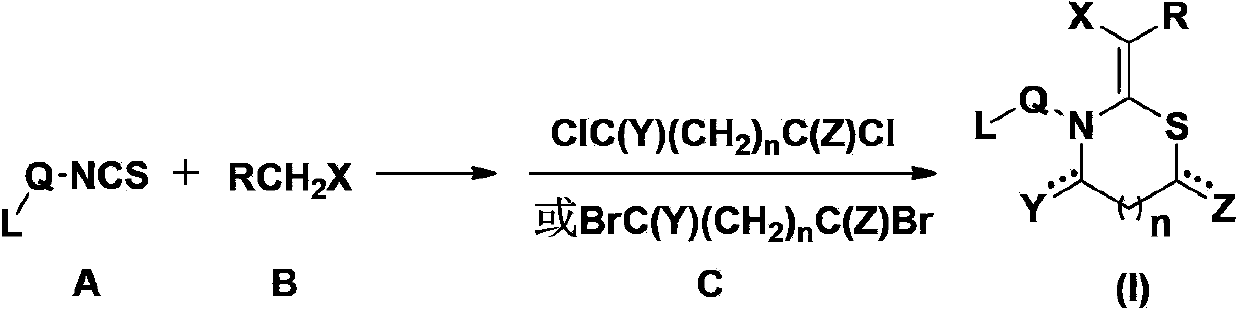
Patents
Literature
169 results about "Cis–trans isomerism" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cis–trans isomerism, also known as geometric isomerism or configurational isomerism, is a term used in organic chemistry. The prefixes "cis" and "trans" are from Latin: "this side of" and "the other side of", respectively. In the context of chemistry, cis indicates that the functional groups are on the same side of the carbon chain while trans conveys that functional groups are on opposing sides of the carbon chain. Cis-trans isomers are stereoisomers, that is, pairs of molecules which have the same formula but whose functional groups are rotated into a different orientation in three-dimensional space. It is not to be confused with E–Z isomerism, which is an absolute stereochemical description. In general, stereoisomers contain double bonds that do not rotate, or they may contain ring structures, where the rotation of bonds is restricted or prevented. Cis and trans isomers occur both in organic molecules and in inorganic coordination complexes. Cis and trans descriptors are not used for cases of conformational isomerism where the two geometric forms easily interconvert, such as most open-chain single-bonded structures; instead, the terms "syn" and "anti" are used.
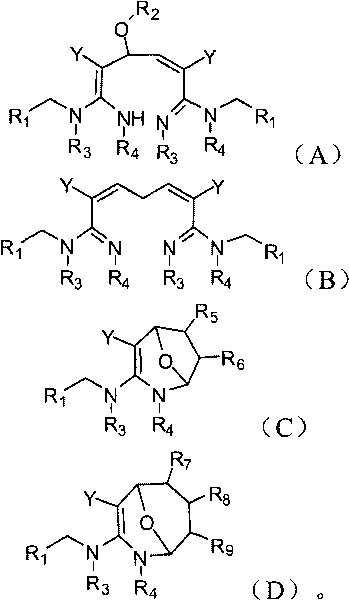
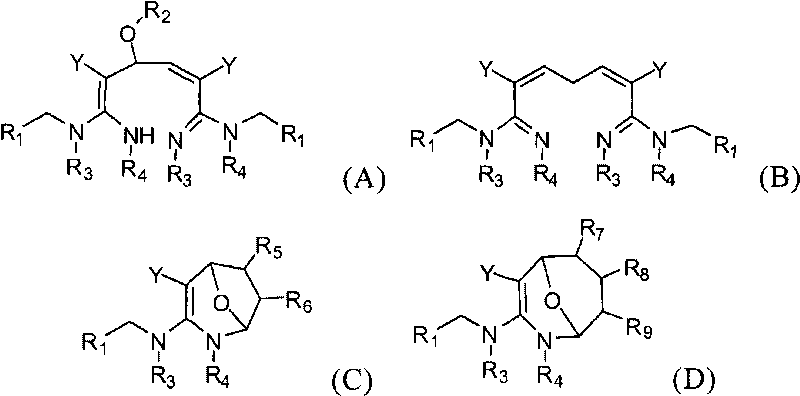
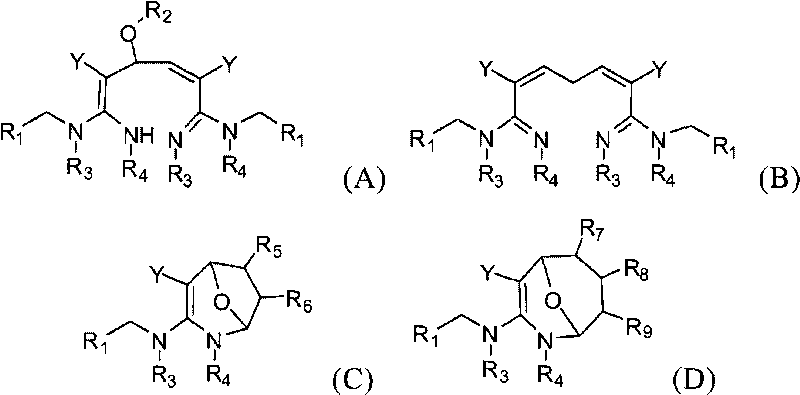
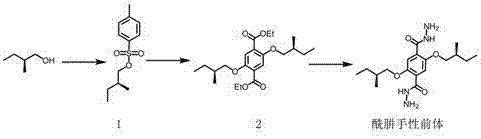
Dialdehyde-built disinsection activity-having nitrogen or oxygen-containing heterocyclic compound and preparation method
Owner:EAST CHINA UNIV OF SCI & TECH
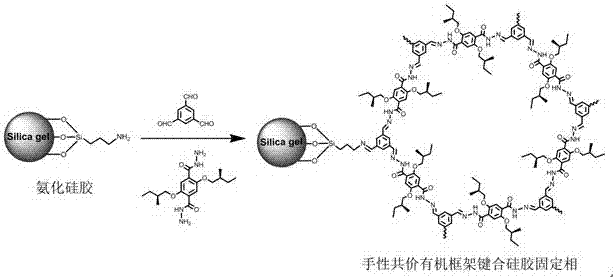
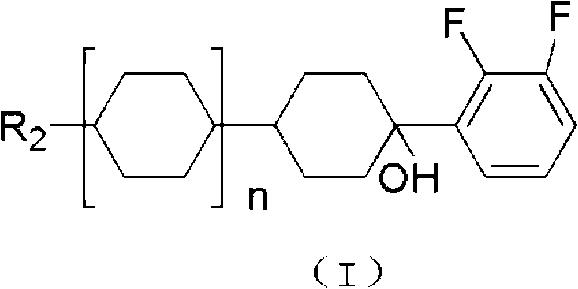
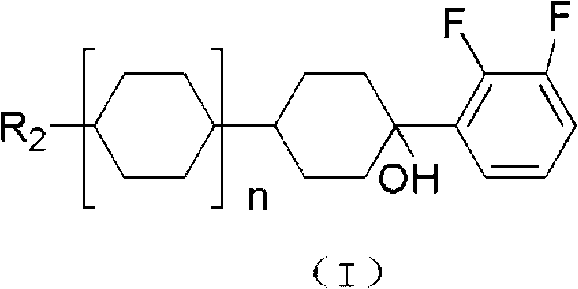
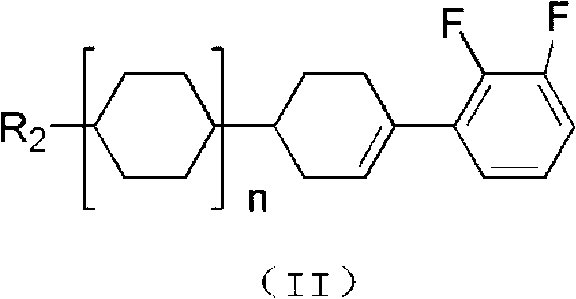
Hydrazone bond-connected chiral covalent organic framework bonded silica gel stationary phase and application thereof
ActiveCN107362785AClear structureEasy to prepareOther chemical processesSolid sorbent liquid separationSilica gelHplc mass spectrometry
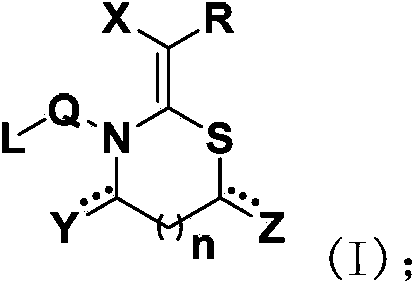
The invention relates to a hydrazone bond-connected chiral covalent organic framework bonded silica gel stationary phase and an application thereof. The stationary phase is prepared by a method comprising the following steps: uniformly mixing a hydrazide chiral precursor, 1,3,5-benzenetricarboxaldehyde and ammoniated silica gel in an organic solvent in an inert atmosphere, carrying out a reaction under the catalysis of acetic acid, and filtering, washing and drying the obtained reaction product to obtain the chiral covalent organic framework bonded silica stationary phase. The structure of the hydrazide chiral precursor is represented by formula (I) shown in the description, a mass ratio of the ammoniated silica gel to the hydrazide chiral precursor is (2-12):1, and molar ratio of the hydrazide chiral precursor to the 1,3,5-benzenetricarboxaldehyde is 1:(0.5-2). The chiral covalent organic framework-bonded silica gel stationary phase is uniform in particle size; and the stationary phase has the advantages of high column efficiency, moderate column pressure and good separation effect on cis-trans isomers and position isomers when used in high performance liquid chromatography, and also has the advantages of definite structure, simple preparation method and good batch reproducibility.
Owner:SOUTH CHINA NORMAL UNIVERSITY
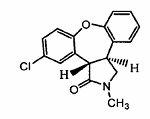
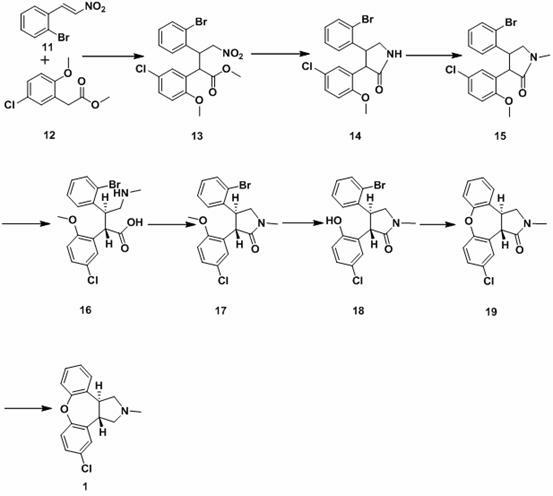
New process for synthesis of asenapine
ActiveCN102229613AReaction transposition effect is goodHigh yieldOrganic chemistryLoop closingMethyl group
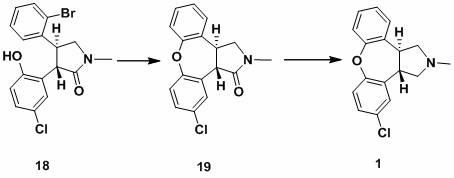
The invention discloses a process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to acondensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:安庆润科生物医药科技有限公司
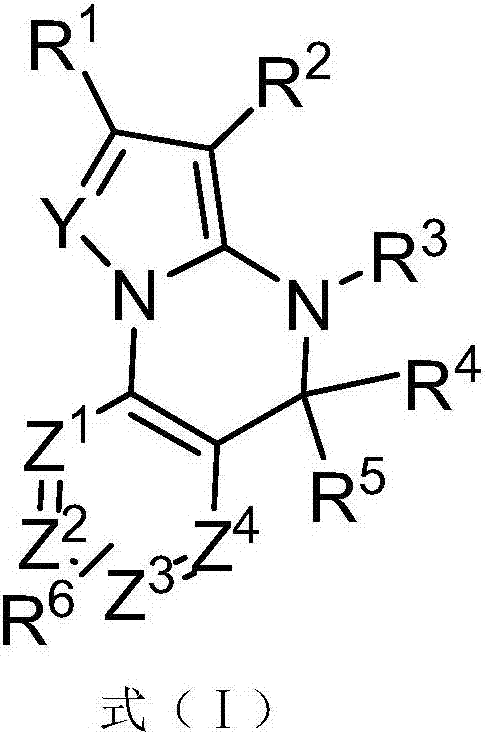
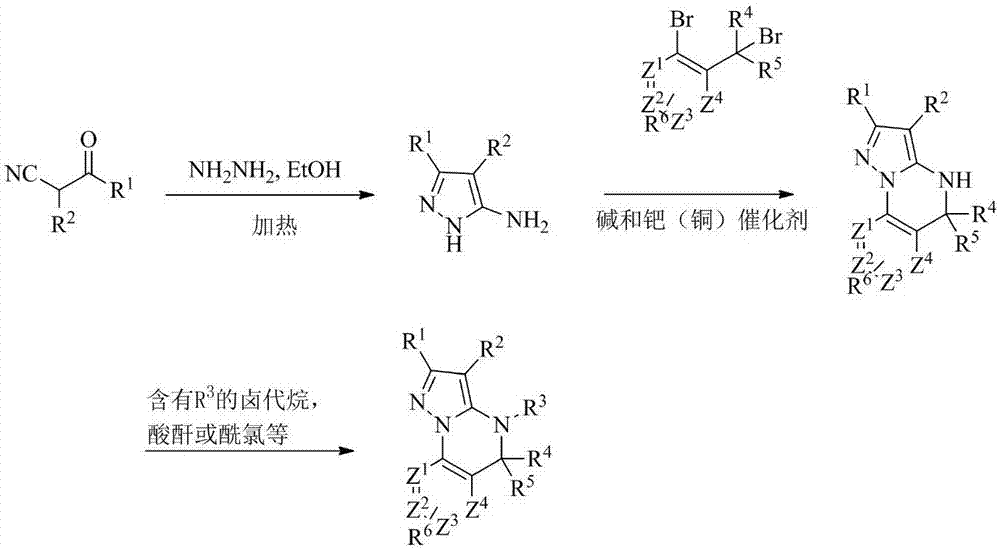
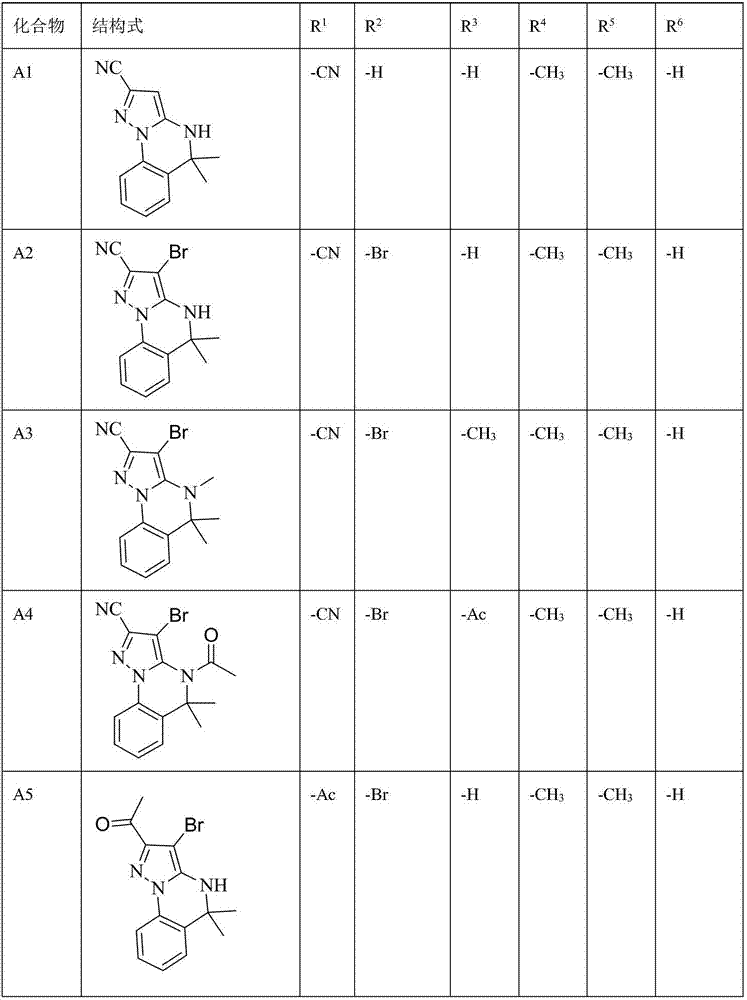
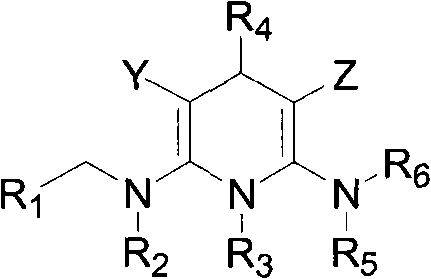
Triazine heterocyclic compound with nematicidal activity as well as preparation method and application of triazine heterocyclic compound
The invention relates to a triazine heterocyclic compound with nematicidal activity as well as a preparation method and application of the triazine heterocyclic compound and particularly discloses a compound as shown in the formula (I) or an optical isomer, cis-trans isomer or pesticidally-acceptable salt of the compound and a preparation method of the compound or the optical isomer, cis-trans isomer or pesticidally-acceptable salt of the compound. The invention also discloses an agricultural composition containing the compound and application of the agricultural composition. The compound has excellent nematicidal activity.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method for side-chain azo-type aqueous polyurethane
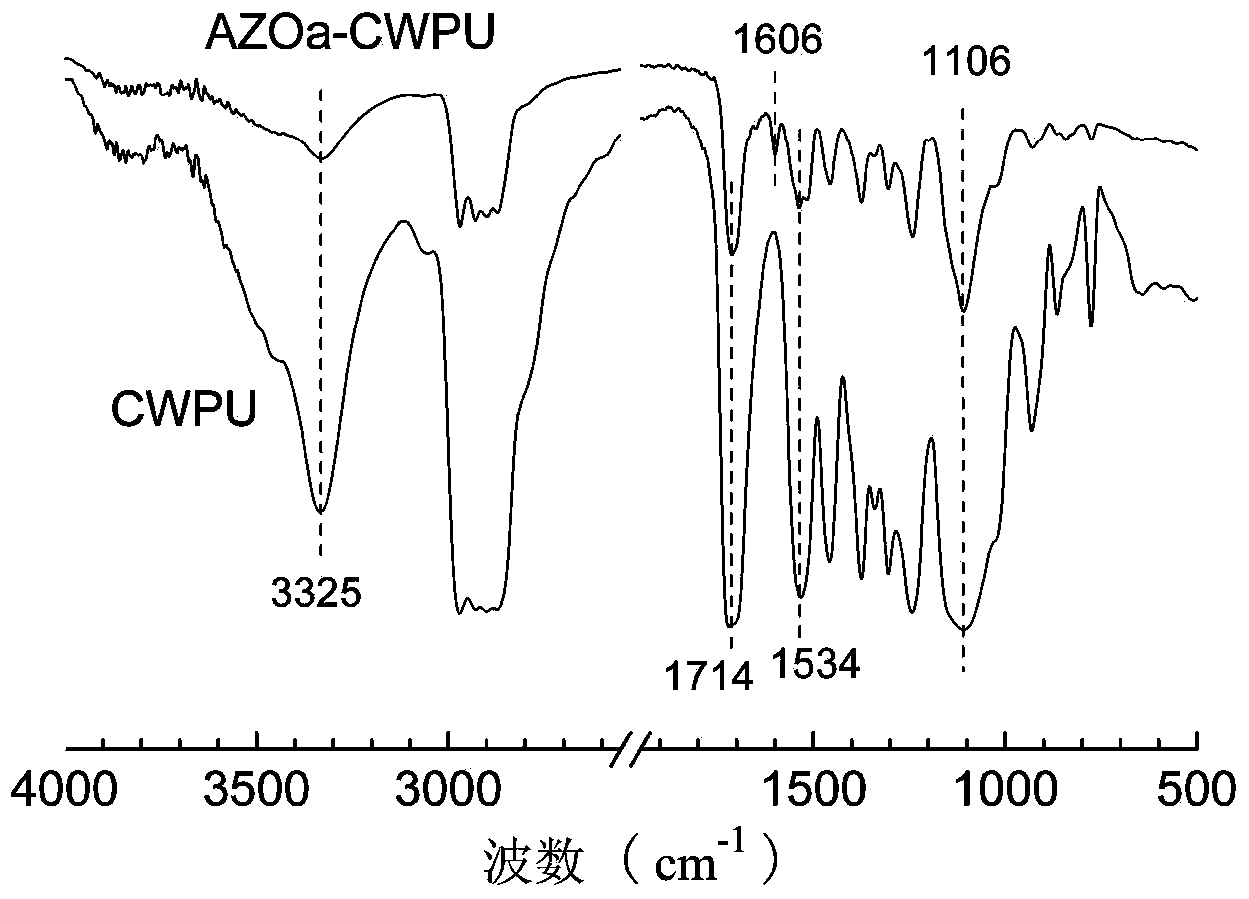
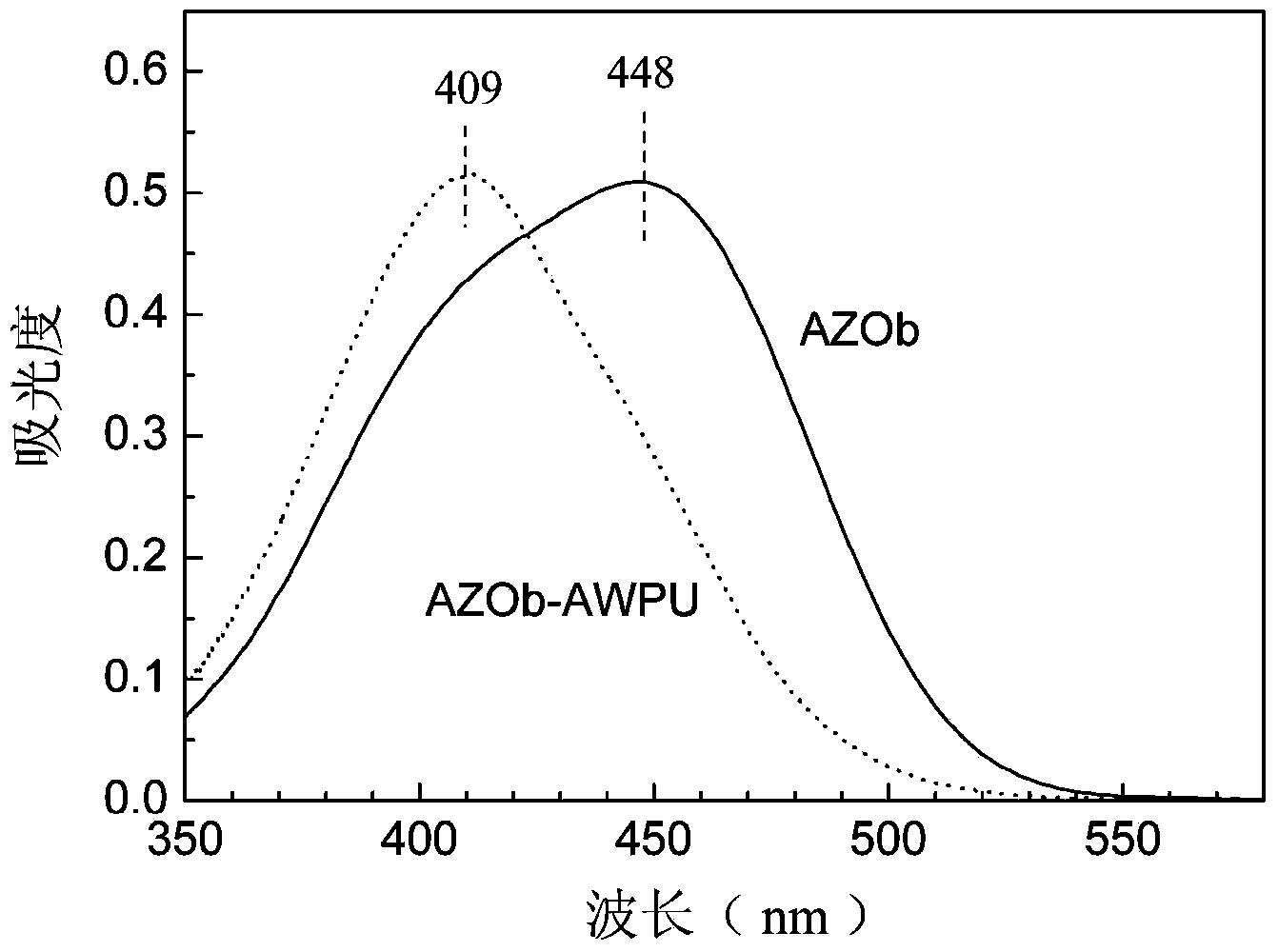
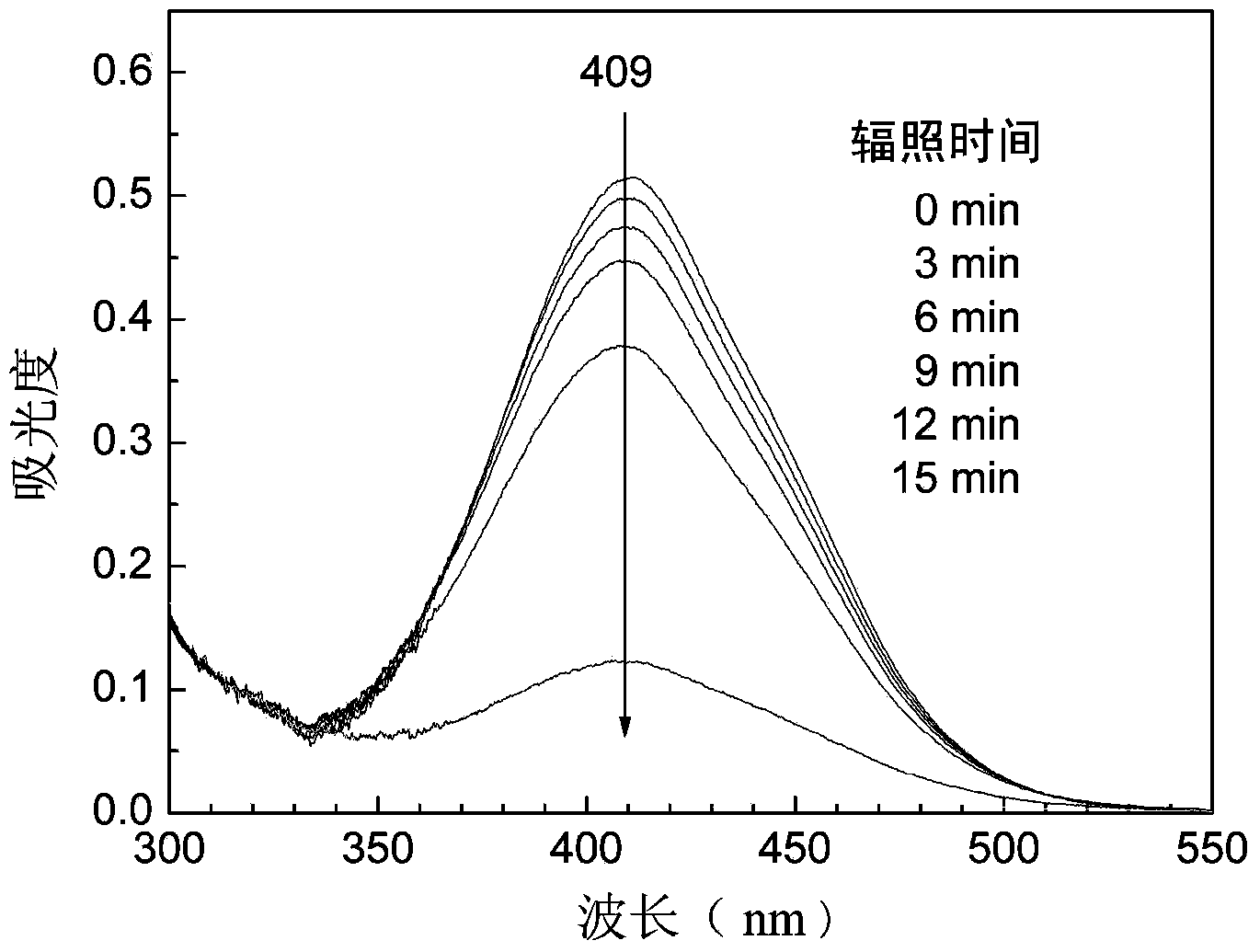
A preparation method for side-chain azo-type aqueous polyurethane is characterized in that a dihydric alcohol containing an azo chromophore and with a symmetric structure is taken as a reactant, and is reacted with a macro-molecule dihydric alcohol and a hydrophilic chain extender for synthesizing cation side-chain azo-type aqueous polyurethane and anion side-chain azo-type aqueous polyurethane. Due to the fact that azo dihydric alcohol is one of the chain extenders, the azo chromophore is in the side chain of the polyurethane molecular chain and other micromolecule chain extenders can be partially or completely replaced, the azo chromophore functional structure has controllable content in the polyurethane molecular chain; the azo chromophore is distributed uniformly and not easy to transfer, and the azo function characteristic can be retained durably; the reversible cis-trans isomerism configuration variation can be generated under the excitation of relatively low energy; and by adjusting the use amount of azo dihydric alcohol, the structure of the polyurethane molecular chain hard segment can be controlled, the side-chain azo-type aqueous polyurethane obtains the azo function characteristics, and also the optical performances, the thermodynamic performances, some functional characteristics and the like of aqueous polyurethane are adjusted.
Owner:宏元(江门)化工科技有限公司 +1
Compound capable of inhibiting IDO (indoleamine 2, 3-dioxyenase), and preparation method and application of compound
ActiveCN106967005AStrong inhibitory activityOrganic active ingredientsSenses disorderHalogenHydrogen atom
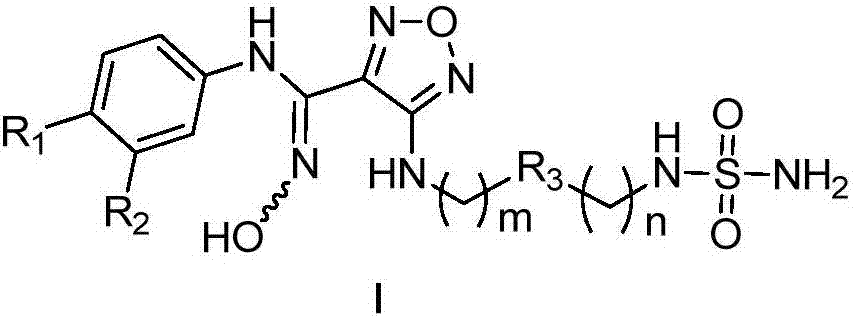
The invention discloses a compound capable of inhibiting IDO (indoleamine 2, 3-dioxyenase), and a preparation method and application of the compound. The structural general formula is shown as below; - is selected from a mixture of a cis-isomer, a trans-isomer or a cis-trans-isomer; R1 and R2 are independently selected from any one of hydrogen atoms, halogen, alkyl, alkoxy or halogenated alkyl; R3 is selected from any one of cyclopentyl, cyclohexyl, piperazinyl or piperidyl; the substitution position of the R3 is (1, 2), (1, 3) or (1, 4); m and n are selected from integers of 0-5 respectively. The compound capable of inhibiting the IDO has high inhibition activity on the IDO, can be used for preparing IDO inhibitors to prevent and / or treat diseases with pathological features of tryptophan metabolic pathways mediated by the IDO, and has quite good application prospect.
Owner:SHANGHAI JOYU PHARMATECH LTD
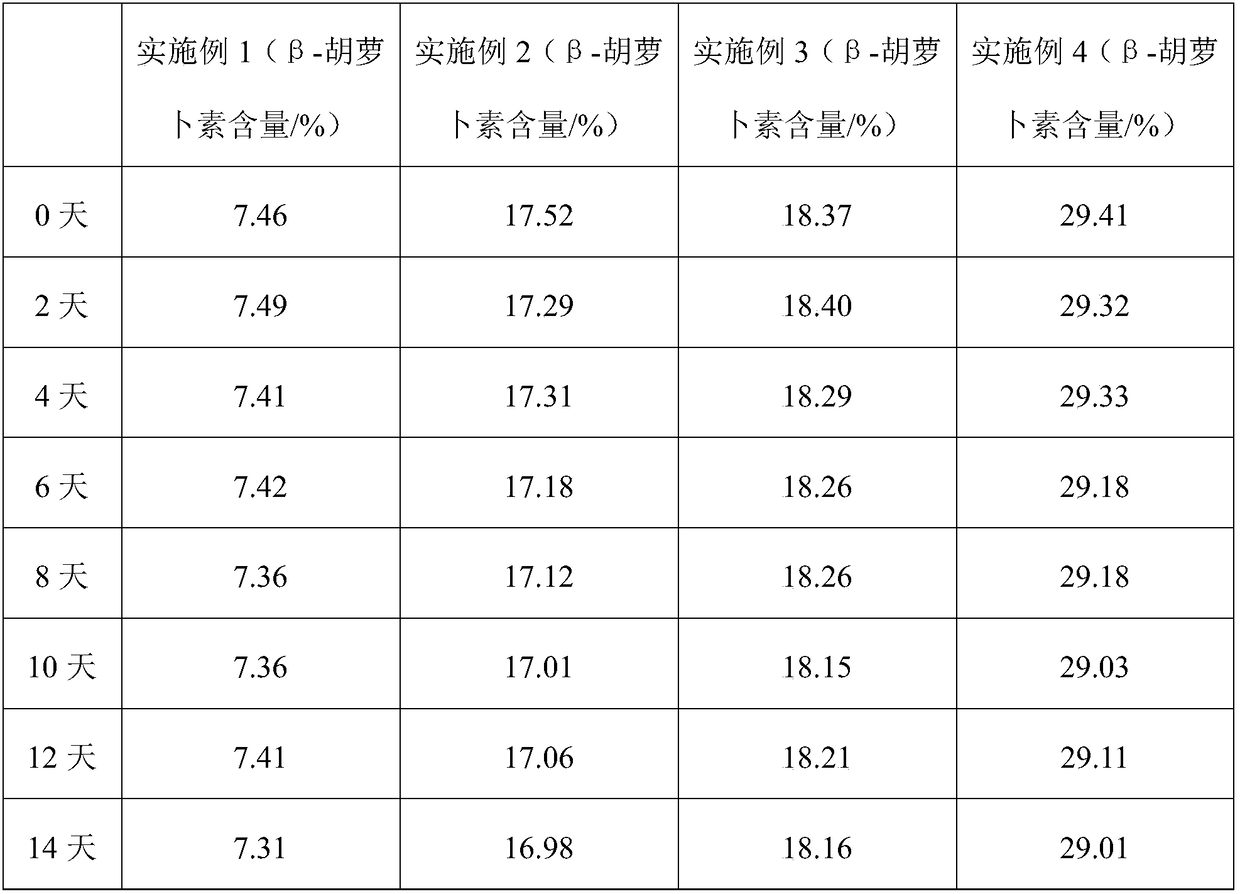
Preparation method of red-colored item beta-carotene preparation with high bioavailability
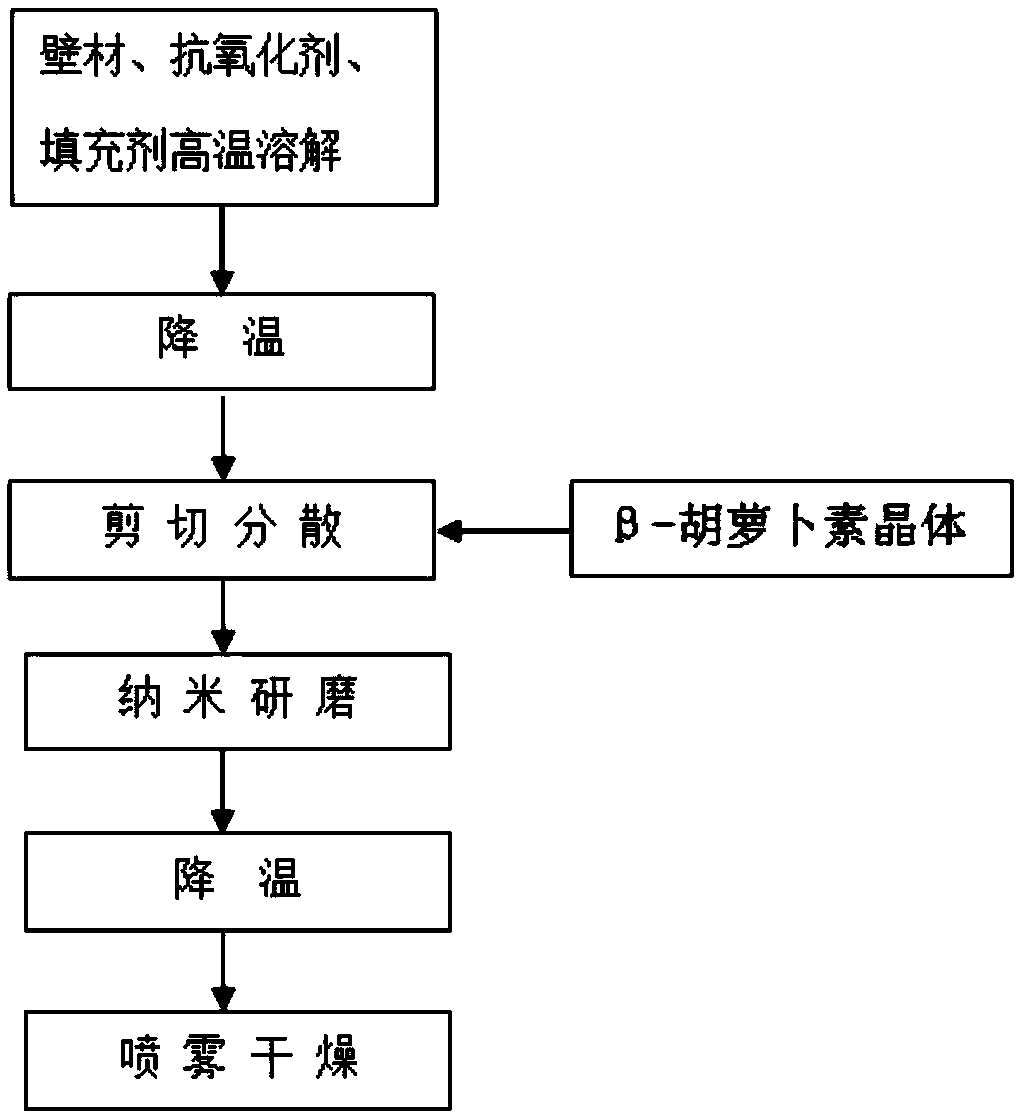
InactiveCN109156827AAvoid cis-trans isomerismNo residueFood shapingNatural extract food ingredientsBeta-CaroteneAntioxidant
The invention relates to a preparation method of a red-colored item beta-carotene preparation with high bioavailability. The preparation method comprises the following steps: firstly, heating a water-soluble wall material, an antioxidant and a filler to fully dissolve the water-soluble wall material, the antioxidant and the filler to form water-soluble colloid, shearing beta-carotene under a low-temperature condition to allow the beta-carotene to be efficiently, quickly and uniformly dispersed in the water-soluble colloid, and finally grinding the beta-carotene through a nano grinding machine.According to the method, no organic solvents are used in the whole steps, and thus the process is environmentally-friendly and is more favorable for food safety. Meanwhile, the method has no high-temperature processes, and no solvent residues remain, so that cis-trans isomerism of the beta-carotene is avoided, and the bioavailability and the safety of the beta-carotene are greatly improved. According to the method, a beta-carotene suspension is cyclically ground through the nano grinding machine so as to finally form nano suspension with a particle size of 100 to 300 nm, so that the beta-carotene is uniformly dispersed in the water-soluble wall material in the form of nano crystals, and the stability and the developing effect are obviously improved; the product is in fresh red; and safe and stable haematochrome is provided for the market.
Owner:武汉星辰现代生物工程有限公司
Preparation method for liquid crystal monomer of o-difluoroalkoxybenzene derivative
ActiveCN102826966AOvercome the disadvantage that the cis structure cannot be isomerizedOvercome the disadvantage of not being able to isomerizeEther preparation by ester reactionsIsomerizationLiquid-crystal display
The invention relates to the field of liquid crystal displays and particularly relates to a preparation method for a liquid crystal monomer of an o-difluoroalkoxybenzene derivative used for liquid crystal displays. According to the preparation method, 1,2-difluorobenzene or 1,2-difluorobromobenzene is used as a raw material and undergoes metallization or a Grignard reaction with a cyclohexyl ketone compound, then dehydration and hydrogenation are carried out to prepare a compound containing cis-trans-isomers, and finally isomerization and oxyalkylation are successively carried out. The method overcomes the disadvantage of incapable isomerization of a cis structure of liquid crystal monomers containing alkoxy groups, increases product yield, reduces production cost, is beneficial for mass production and has a great application prospect.
Owner:VALIANT CO LTD
Fused heterocycle compounds containing pyrazolone rings and applications thereof
ActiveCN108129481AEnhance killing activityGood killing effectBiocideOrganic chemistryPyrazoloneRed imported fire ant
The invention provides a type of fused heterocycle compounds containing pyrazolone rings, optical isomers, cis-trans-isomers or pharmaceutically acceptable salts of fused heterocycle compounds and applications of the fused heterocycle compounds, and the optical isomers, cis-trans-isomers or pharmaceutically acceptable salts of the fused heterocycle compounds. The fused heterocycle compounds have astructure represented by a formula (I) (shown in the description). The fused heterocycle compounds containing the pyrazolone rings, and the optical isomers, the cis-trans-isomers or the pharmaceutically acceptable salts of the fused heterocycle compounds have high killing activity to agriculture and forestry pests, health pests and the like, furthermore, and the compounds have a delayed action effect to pests such as red imported fire ants, so the pests can carry the drug to a nest, and the whole nest and an queen ant can be well killed; and the compounds have very good application prospects.
Owner:SOUTH CHINA AGRI UNIV
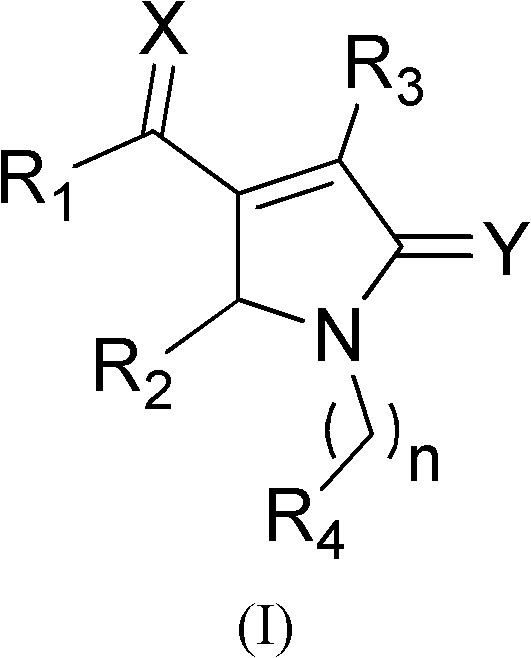
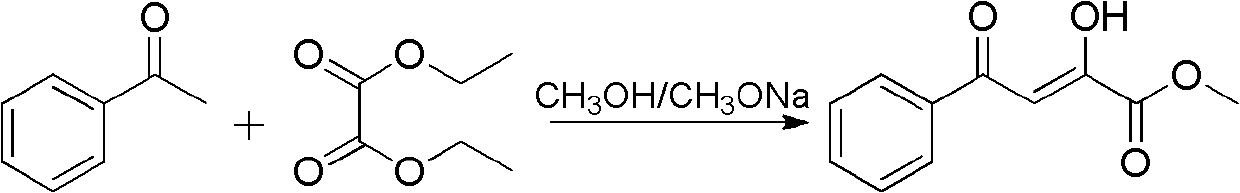
Pyrrylketone compound and application thereof as drug
InactiveCN102603717AGood antitumor activityOrganic active ingredientsOrganic chemistryMedicinal chemistryPyrrole
The invention relates to the technical field of medicines, particularly a pyrrylketone compound and application thereof as a drug. The structure of the compound is disclosed as Formula (I), comprising optical isomer, raceme, cis-trans-isomer and any combination or medicinal salts thereof. The compound provided by the invention can be used as a micromolecular inhibitor for p53-MDM2 / X protein interactions, and can be used for preparing the antineoplastic drug.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
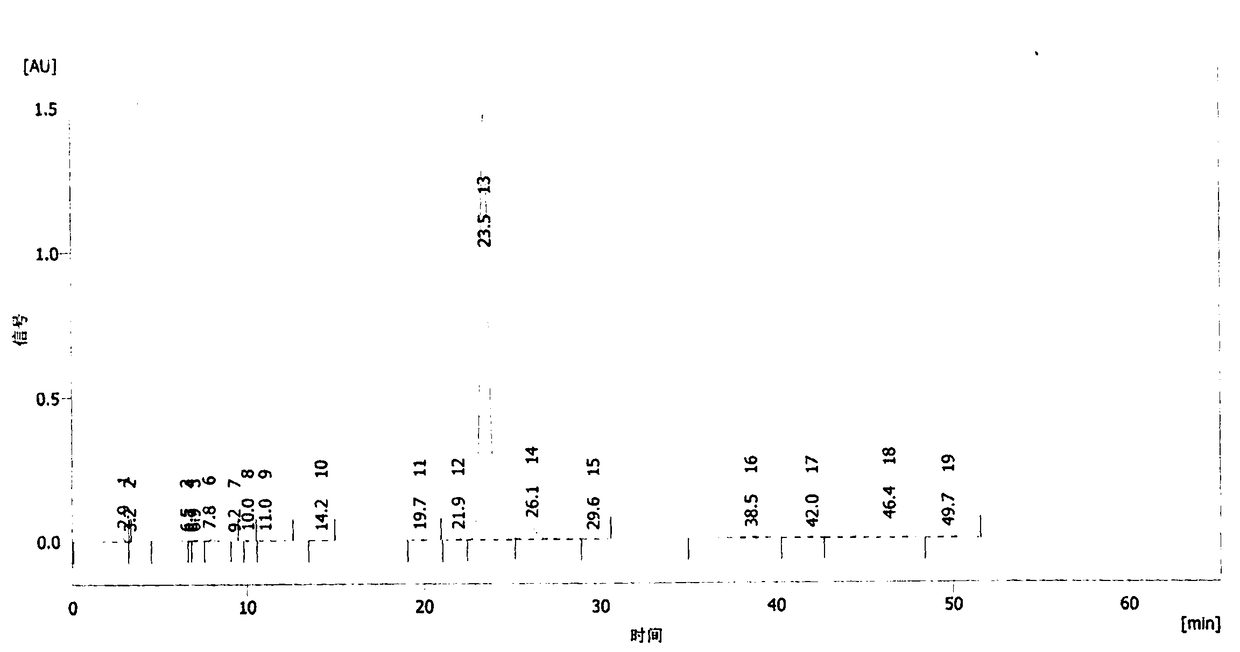
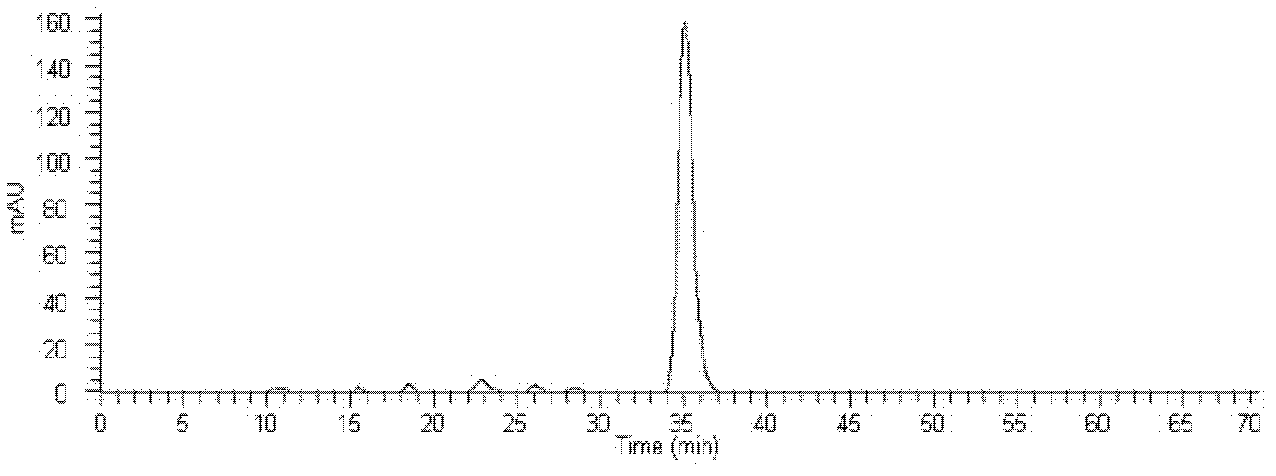
HPLC-MS detection method of xanthophylls cis-trans-isomers in xanthophylls products
InactiveCN103018351AMeasurement precisionAssay stabilityComponent separationInjection volumeIsomerization
The invention belongs to the field of analysis technology, and relates to an HPLC-MS detection method of xanthophylls cis-trans-isomers in xanthophylls products. The method is characterized by performing a light iodine isomerization reaction for the all-trans xanthophylls to obtain the cis-isomeride of the xanthophylls; using a YMC Carotenoid C30 chromatographic column to substantially separate the xanthophylls isomer, wherein a mobile phase is methanol / water = 98 / 2, a time is 70 minutes, a flow velocity rate is 1.0 mL / min, a DAD detector is used, a column temperature is 25 DEG C, an injection volume is 20 [mu]l and a detection wavelength is 450 nm; and using a positive ion mass spectrometry (APCI / MS), wherein a flow rate of the components from the chromatographic column into the mass spectrometer is 10 [mu]L / min, a scanning range m / z is 200 to 800, a capillary temperature is 150 DEG C, a vaporization temperature is 450 DEG C, a capillary voltage is 10 V, and a flow rate of dry gases is 8 mL / min. According to information of the mass spectrum and the spectrum, the xanthophylls isomers are respectively determined as all trans, 9-cis, 9'-cis, 13-cis and 13'-cis xanthophylls. The analysis method is rapid and effective, good in reproducibility and high in recovery rate, and can quantitatively analyze content of the xanthophylls cis-trans-isomers in the xanthophylls products.
Owner:NORTHEAST FORESTRY UNIVERSITY
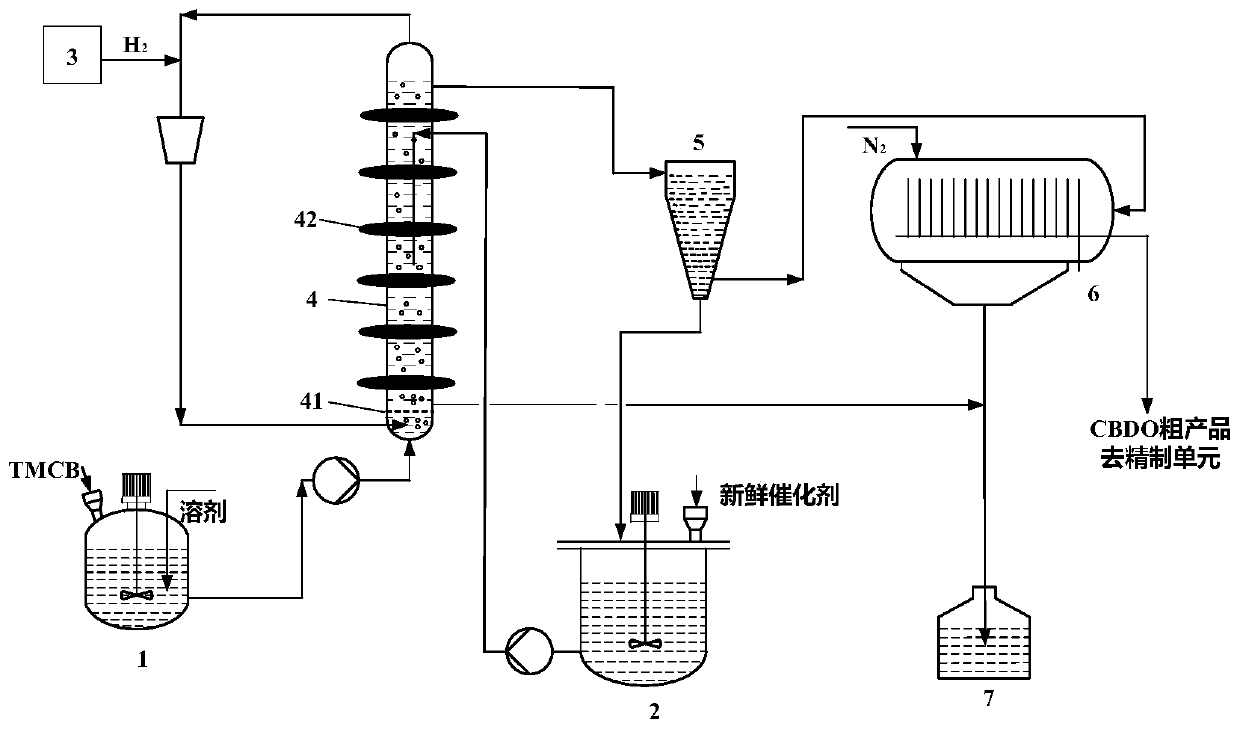
Catalyst, preparation method thereof, and apparatus and method for preparing 2,2,4,4-tetramethyl-1,3-cyclobutanediol
InactiveCN110124674AImprove conversion rateHigh yieldOrganic compound preparationHydroxy compound preparationKetoneMethyl group
The invention relates to the field of production of 2,2,4,4-tetramethyl-1,3-cyclobutanediol, and concretely relates to a catalyst, a preparation method thereof, and an apparatus and a method for preparing 2,2,4,4-tetramethyl-1,3-clobutanediol. The catalyst comprises a main active metal element, a metalloid element, a carrier and an optional coactive metal element, the main active metal element isone or more of Fe, Co and Ni, the metalloid element is B and / or P, and the coactive active metal element is one or more of Co, Ru, Pd, Rh, Ir, and Pt; and the content of the main active metal elementin the catalyst is 1-70 wt%. The catalyst, the apparatus and the preparation methods of the invention are used to realize high-efficiency conversion of ketones, improve the cis-trans isomer molar ratio of the 2,2,4,4-tetramethyl-1,3-cyclobutanediol product and reduce the wearing and consumption of the catalyst.
Owner:ZHEJIANG HENGYI PETROCHEMICAL RES INST CO LTD
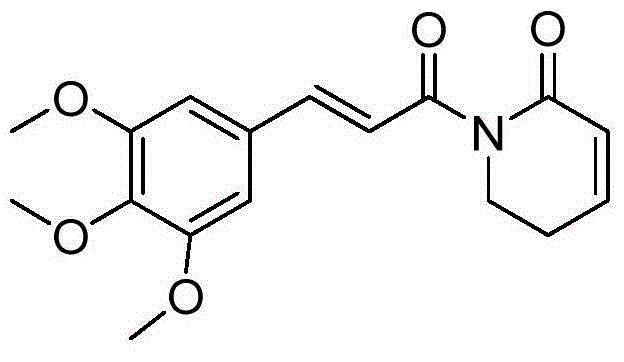
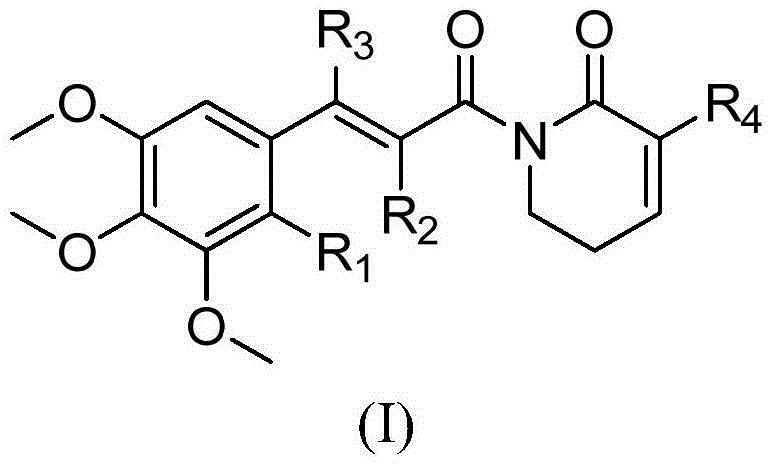
Piplartine analogue, and preparation method and application thereof
The invention relates to the technical field of medicines. Piplartine is alkaloid extracted from perennial herbaceous vine piper longum of piperaceae. The invention provides a Piplartine analogue, which comprises a cis-trans-isomer, and any arbitrary mixture in those forms or medicinal salt thereof, and the structural formula of the Piplartine analogue is shown in a general formula (I). The invention also provides a preparation method of substituted Piplartine compounds, and an application thereof in preparation of an antitumor medicament.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
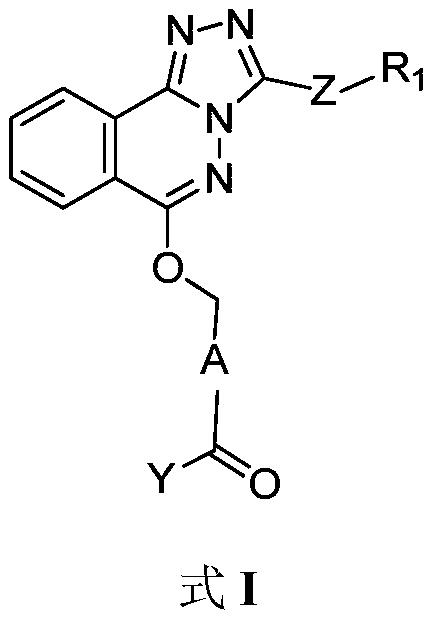
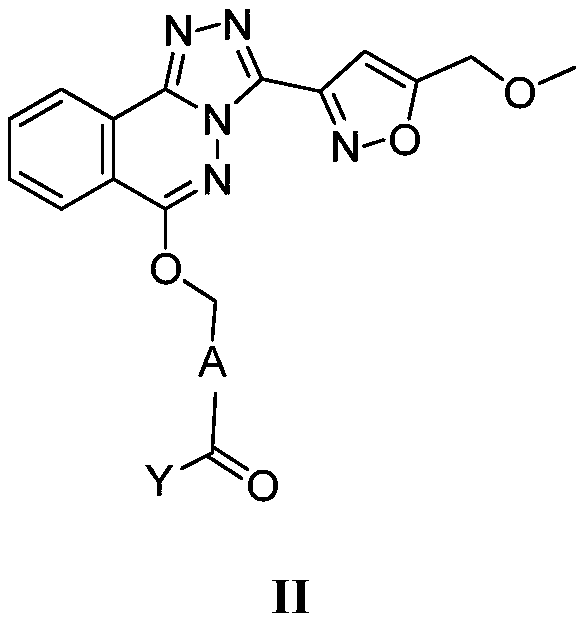
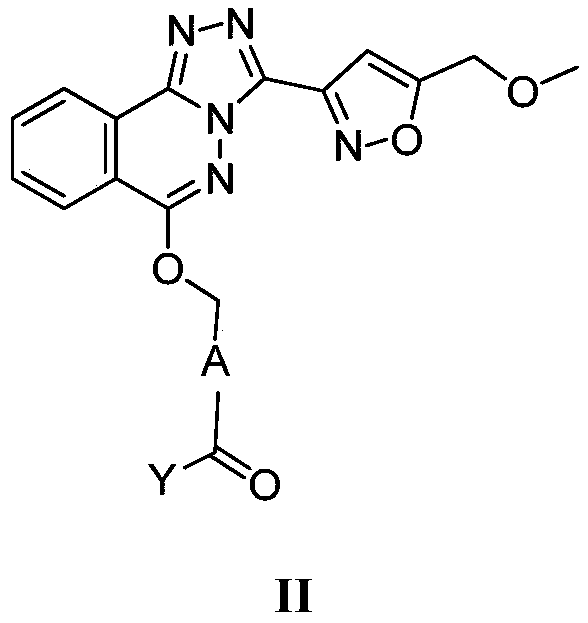
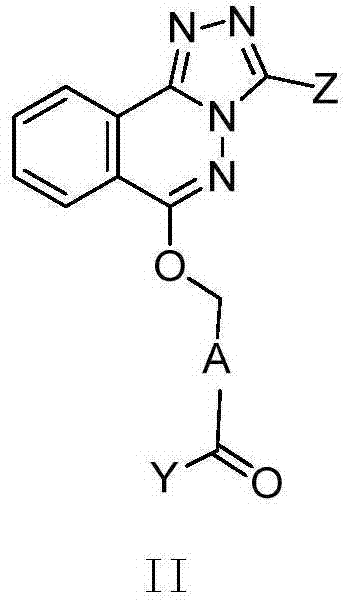
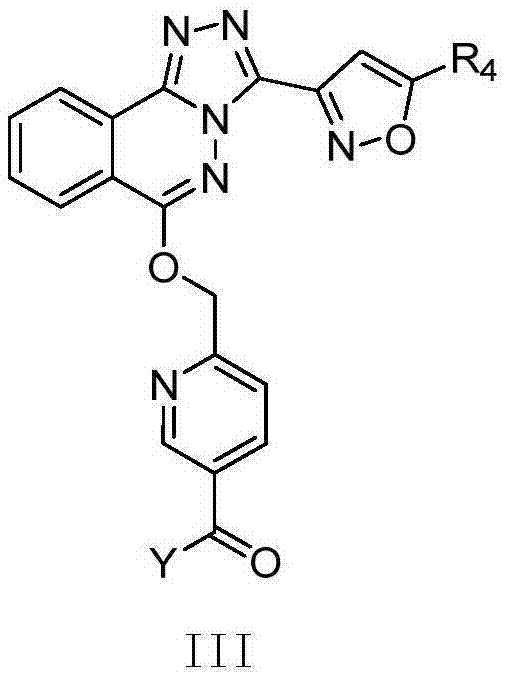
Phthalazine isoxazole alkoxy derivative, preparation method, pharmaceutical composition and uses thereof
ActiveCN110256440AGood inverse agonismOrganic active ingredientsNervous disorderEnantiomerDiastereomer
The present invention discloses a phthalazine isoxazole alkoxy derivative, a preparation method, a pharmaceutical composition and uses thereof, and provides a compound represented by a formula I, a cis-trans isomer, an enantiomer, a diastereomer, a racemate, a solvate, a hydrate, a pharmaceutically acceptable salt or a prodrug thereof. According to the present invention, the compound has good inverse agonistic effect on alpga5-GABAA.
Owner:SHANGHAI SIMR BIOTECHNOLOGY CO LTD +1
Fused heterocyclic compounds and application thereof
ActiveCN108003162AGood killing effectEnhance killing activityBiocideOrganic chemistryApis ceranaChemical compound
The present invention discloses fused heterocyclic compounds as well as optical isomers, cis-trans isomers or agrochemically acceptable salts thereof and an application of a composition. The fused heterocyclic compounds have a structure shown in a formula I in the description. The fused heterocyclic compounds as well as the optical isomers, the cis-trans isomers or the agrochemically acceptable salts thereof provided by the present invention have high killing activity against agricultural and forestry pests, animal parasitic pests, sanitary pests and the like, delayed effects on invasive solenopsis invicta, and lower toxicity to environmental organisms such as apis cerana and bombyx mori.
Owner:SOUTH CHINA AGRI UNIV
Nitrogen-containing heterocyclic compound with pesticidal activity, preparation and application thereof
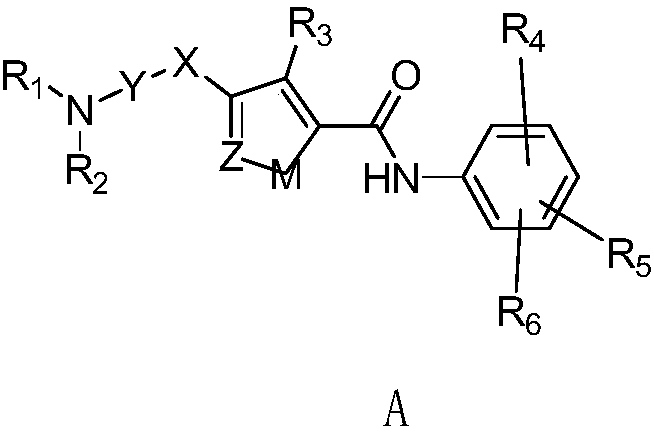
The invention relates to a compound with a general formula (A) or an optical isomer, cis-trans isomer or pesticide science-acceptable salt of the compound and a preparation method thereof. The invention also relates to an agricultural composition containing the compound or the optical isomer, cis-trans isomer or pesticide science-acceptable salt of the compound and the application of the agricultural composition. The compound and derivatives thereof have high pesticidal activity on homoptera, lepidoptera and other agricultural and forest pests, such as aphids, plant hoppers, whiteflies, leafhoppers, thrips, cotton bollworms, cabbage caterpillars, diamond back moths, prodenia lituras and armyworms.
Owner:EAST CHINA UNIV OF SCI & TECH
3,4-dihydropyridine-2-ketone heterocyclic compound and application thereof
The invention relates to a nicotine compound and application thereof. The nicotine compound is a 3,4-dihydropyridine-2-ketone heterocyclic compound or an optical isomer, a cis-trans isomer or acceptable salt on the pesticide science thereof. The nicotine compound in the invention has higher insecticidal activity on agroforestry pests, sanitary pests and pests harming the health of animals.
Owner:EAST CHINA UNIV OF SCI & TECH
Phthalazine derivatives, and preparation method, pharmaceutical composition and application thereof
The invention provides compounds as shown in a general formula (I) which is described in the specification and cis-trans-isomers, enantiomers, diastereomers, racemes, solvates, hydrates, pharmaceutically acceptable salt and esters thereof. The invention further provides preparation method for the compounds, pharmaceutical compositions of the compounds and application of the compounds as modulators for an alpha5-GABAA receptor. T, Z, A and Y in the formula (I) are as defined in the specification.
Owner:SHANGHAI SIMR BIOTECHNOLOGY CO LTD +1
Preparation and application of 1,2,3-3H pyridine-heterocyclic compound
The invention relates to preparation and application of a 1,2,3-3H pyridine-heterocyclic compound. Specifically, an N heterocycle-contained or open ring-contained compound as shown in a general formula (A) in the specification, or an optical isomer and a cis-trans isomer thereof, or agriculturally-pharmaceutically acceptable salts are provided. The invention also relates to an agricultural composition containing the compound, or the optical isomer and the cis-trans isomer thereof or agriculturally-pharmaceutically acceptable salts, and the application of the agricultural composition. The compound and the derivatives thereof have extremely high insecticidal activity on agriculture and forestry pests, sanitary pests and pests harming animal health. The general formula (A) is shown in the specification.
Owner:EAST CHINA UNIV OF SCI & TECH
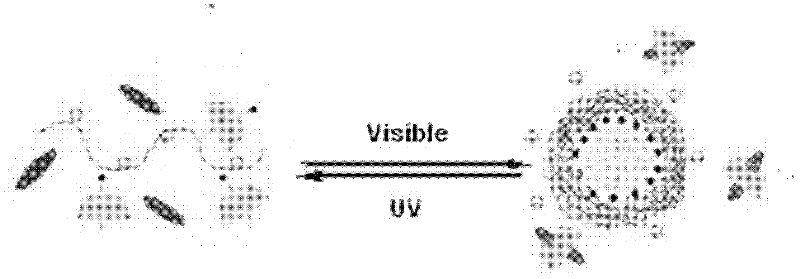
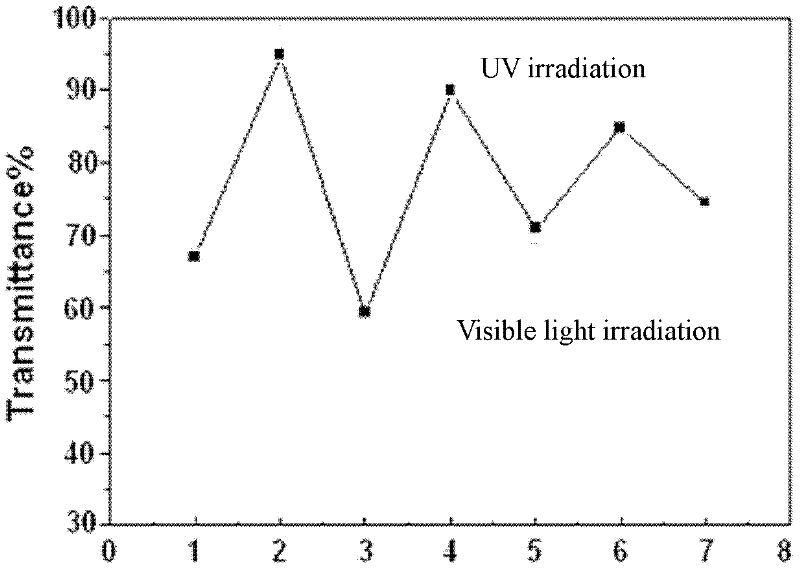
Photoresponsive polymer microsphere system and preparation method thereof
The invention discloses a photoresponsive polymer microsphere system and a preparation method thereof. The system is a solution containing cyclodextrin, an azo compound having cis-trans isomerism respectively under the irradiation of ultraviolet light and visible light, and amphiphilic polyelectrolyte containing a hydrophobic alkyl chain. Polymer microspheres are generated when the system is under the irradiation of visible light, and the polymer microspheres in the system are decomposed under the irradiation of ultraviolet light. The preparation method comprises the following steps of: dissolving the amphiphilic polyelectrolyte in a solvent; completely stirring a microsphere solution; and then adding cyclodextrin and stirring for dissolving cyclodextrin; and finally adding the azo compound to obtain the photoresponsive polymer microsphere system after the azo compound is dissolved. The photoresponsive polymer microsphere system provided by the invention has the characteristics that the preparation conditions are moderate, the formation and decomposition of microspheres are reversible, quick and controllable. The photoresponsive polymer microsphere system has a bright application prospect in the biomedicine field such as medicine transfer, medicine slow release and the like, as well the anti-counterfeiting filed relating to trademarks, bills, even military industry, national defense and the like.
Owner:SICHUAN UNIV
Process for the preparation and purification of cis-2-methylspiro(1,3-oxathiolane-5,3')quiniclidine hydrochloride
InactiveUS20090182146A1Easily scalableSubstantial enrichment of the cis-isomerOrganic chemistryOrganic solventMedicinal chemistry
An industrially acceptable process for the preparation and purification of cis-2-methylspiro(1,3-oxathiolane-5,3′)quiniclidine from a cisitrans mixture of isomers. Treatment of the mixture with an organic sulfonice acid generates a less soluble acid addition salt that is enriched in the cis-isomer. Recrystallization or pulping using various organic solvents allows for enrichment of the cis-isomer by filtration. These new sulfonic acid salts of the cis-isomer of 2-methylspiro(1,3-oxathiolane-5,3′)quiniclidine prepared according to the present invention could be further converted into the hydrochloride salt by any known procedures such as treatment with a base and then hydrochloric acid salt formation or exchange of the sulfonic acid salt with hydrochloric acid.
Owner:APOTEX PHARMACHEN INC
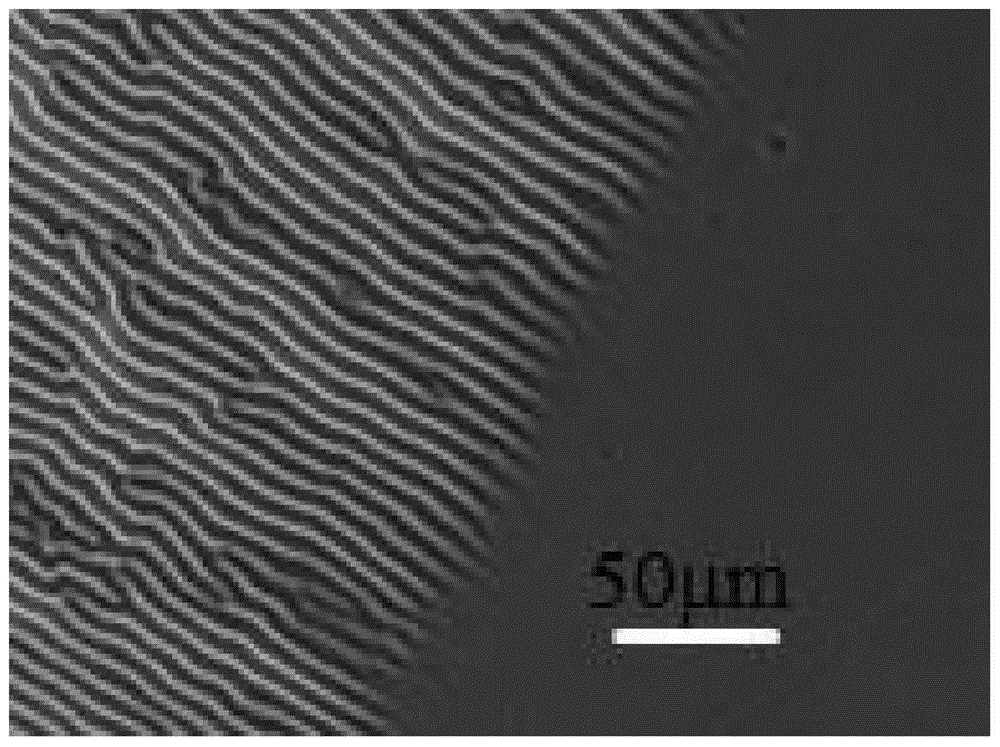
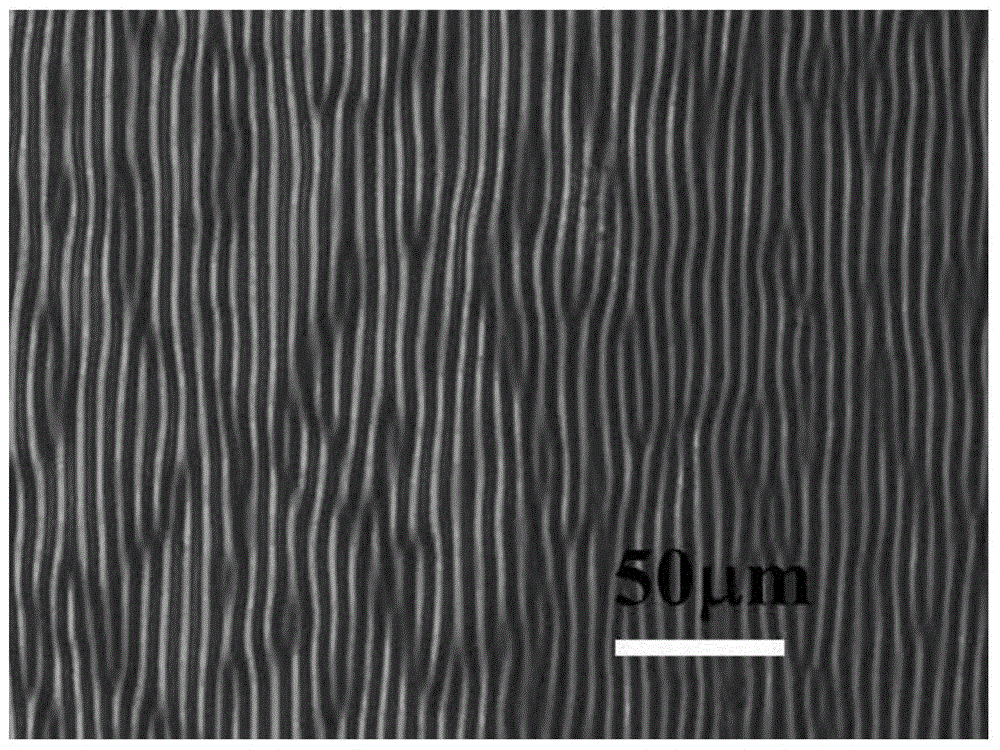
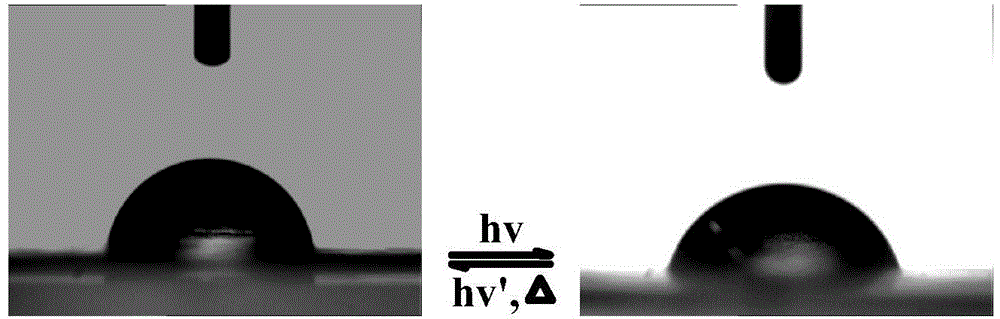
Method for stopping wrinkles from being formed on surface of azobenzene thin film by light illumination
The invention discloses a method for stopping wrinkles from being formed on the surface of an azobenzene thin film by light illumination. The formation of the wrinkles on the surface of the azobenzene thin film is stopped by the light illumination through utilizing special light-induced trans-cis isomerization characters of azobenzene. The method comprises the following steps: firstly, spin-coating a polydimethylsiloxane (PDMS) substrate subjected to oxygen plasma activation treatment with a tetrahydrofuran solution of an azobenzene polymer (PAzo), so as to form a PDMS / PAzo soft-hard composite system; drying and discharging a tetrahydrofuran solvent. Under common conditions, the wrinkles are formed by the PDMS / PAzo composite system under the action of external stress. When a stress effect is applied, the light illumination is carried out, so that the wrinkles can be effectively prevented from formation. By controlling the relative size of illumination light intensity and external stress, whether the wrinkles are formed by the PDMS / PAzo double-layered system or not can be regulated and controlled. The method is simple and convenient, and is clean and effective; the method has a wide application prospect in the aspects of preventing a material from being invalid and prolonging the service life of the material in the field of composite materials.
Owner:TIANJIN UNIV
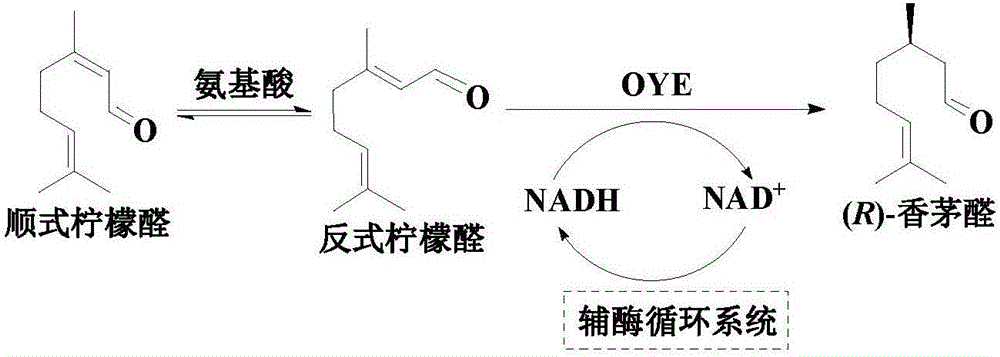
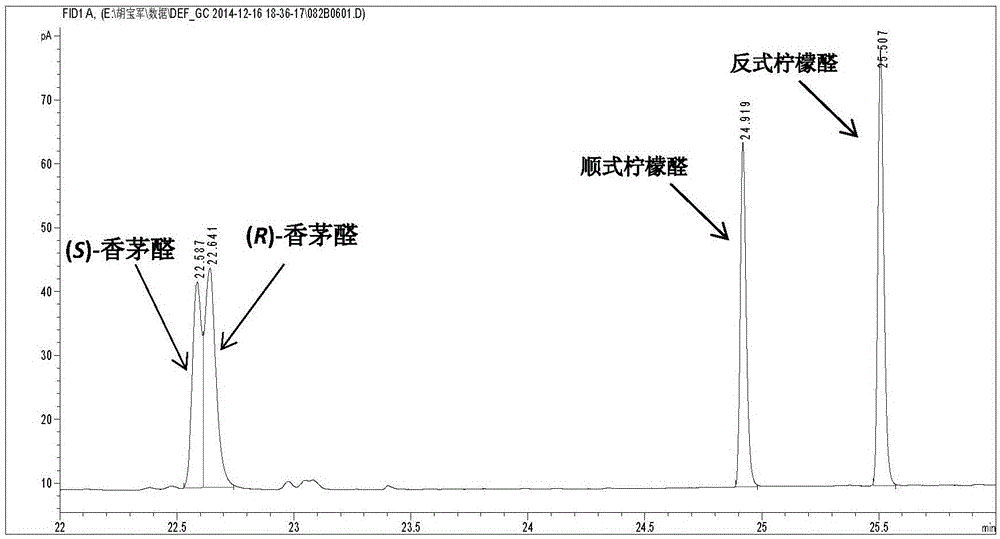
An enzymatic citral asymmetric reduction method capable of increasing optical purity of (R)-citronellal
An enzymatic citral asymmetric reduction method capable of increasing optical purity of (R)-citronellal is disclosed, namely a method coupling an amino-acid-catalyzed citral cis-trans isomerization reaction and a citral asymmetric hydrogenation reaction catalyzed by a saccharomyces cerevisiae enol reductase OYE1 to increase the optical purity of the (R)-citronellal that is a product of citral hydrogenation. When citral cis-trans isomers are subjected to the asymmetric hydrogenation reaction catalyzed by the saccharomyces cerevisiae enol reductase OYE1 to synthesize the (R)-citronellal, the (R)-citronellal is derived from trans-citral, (S)-citronellal is derived from cis-citral, and the catalysis speed for the trans-citral is higher than that for the cis-citral. Through coupling with the amino-acid-catalyzed citral cis-trans isomerization reaction, a part of the cis-citral is converted into the trans-citral, thus greatly increasing the ee value of the product that is the (R)-citronellal. In a catalytic system having a volume of 10 mL, 100 mg / mL of glycine is added, after 50 mM citral is subjected to a catalytic reaction for 4 h, the ee value of the (R)-citronellal is 65.4%, and is increased by 48.7% when being compared with the ee value of (R)-citronellal when the cis-trans isomerization reaction is not coupled.
Owner:ZHEJIANG UNIV OF TECH
N, S-containing heterocyclic compound with nematicidal activity, preparation method and application thereof
The invention relates to an N, S-containing heterocyclic compound with nematicidal activity, a preparation method and application thereof. Specifically, the invention discloses a compound as formula (I) or optical isomers, cis-trans-isomers of the compound or agricultural pharmacology acceptable salts, and the preparation method thereof. The invention also discloses agricultural compositions containing the compound and application thereof. The compound has excellent nematicidal activity. (formula I).
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method and application of mycotoxin immunosensor based on graphdiyne-toluidine blue composite material
ActiveCN106053789ASpecific surface area enlargementFacilitates electron transferMaterial electrochemical variablesMycotoxinGraphite
The invention relates to a preparation method and an application of a mycotoxin immunosensor based on a graphdiyne-toluidine blue composite material, and belongs to the technical field of novel biosensor sensing detection. Due to the sp hybridization structure of graphdiyne, graphdiyne has the advantages of no cis-trans isomer and high conjugation property. At the same time, the good [phi]-[phi] conjugation effect between toluidine blue and graphdiyne is utilized, the single molecules can be fixed firmly therefore, and the signal error caused by instable combination is reduced. So the provided mycotoxin immunosensor based on a graphdiyne-toluidine blue composite material can sensitively detect mycotoxin.
Owner:UNIV OF JINAN
Preparation method of aqueous polyurethane based on anionic azo hydrophilic chain extender
ActiveCN104861148AImprove hydrophilic abilityReduce processPolyurea/polyurethane coatingsEmulsionSide chain
The invention discloses a preparation method of aqueous polyurethane based on an anionic azo hydrophilic chain extender. According to the preparation method, the anionic azo hydrophilic chain extender containing azo color radicals is utilized for partially or completely replacing a normal hydrophilic chain extender, so as to prepare azo anionic aqueous polyurethane; the problem that the content of hydrophilic groups in a polyurethane system is greatly limited or reduced by a traditional copolymerization method cannot be caused by the addition of the color radicals. According to the azo anionic aqueous polyurethane prepared by virtue of the preparation method, the azo color radicals are located at side chains of polyurethane molecular chains, the azo hydrophilic chain extender with strong hydrophily is distributed on the surfaces of emulsified sphere particles to form a stable double electric layer structure in a self-emulsifying process, the reversible cis-trans isomerism change can be caused by virtue of relatively low energy excitation, the quantity of the azo groups linked into aqueous polyurethane is large, and the change of polyurethane emulsion particle size caused by ultraviolet irradiation shows that functional materials can be used as ultraviolet light responsive nano carriers.
Owner:UNIV OF SCI & TECH OF CHINA +1
Sulfonamide-arylamide compound, and medicinal application of same to treatment of hepatitis B
The invention relates to a sulfonamide-arylamide compound, and medicinal application of the same to treatment of hepatitis B. Specifically, the invention discloses a compound having a structure as shown in a formula (A) and applicable as a HBV replication inhibitor, or a stereoisomer, cis-trans isomer or tautomer thereof, or a pharmaceutically acceptable salt, hydrate or solvate thereof. Each group in the formula (A) is as defined in the specification. The invention further relates to a pharmaceutical composition comprising the above compound and application of the pharmaceutical composition to the treatment of hepatitis B.
Owner:SHANGHAI LONGWOOD PHARMA
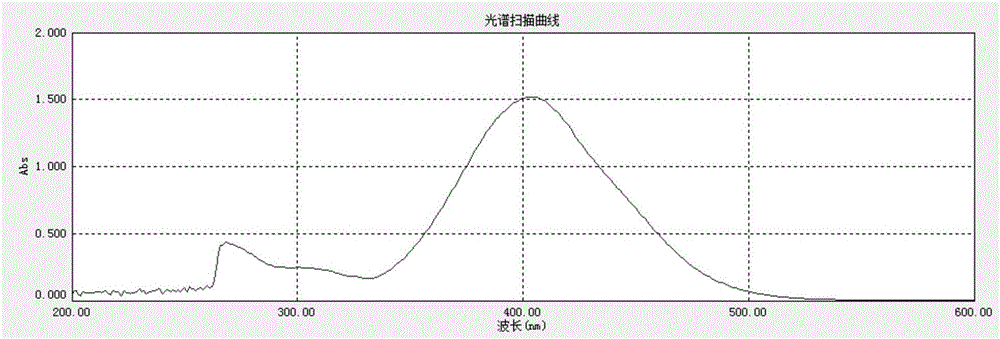
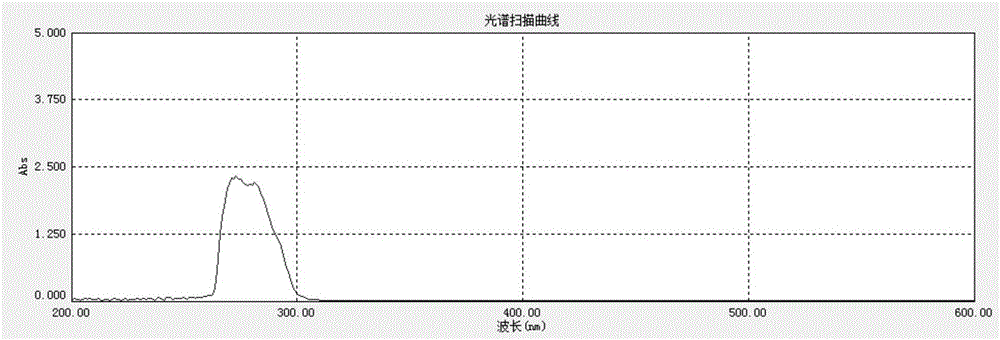
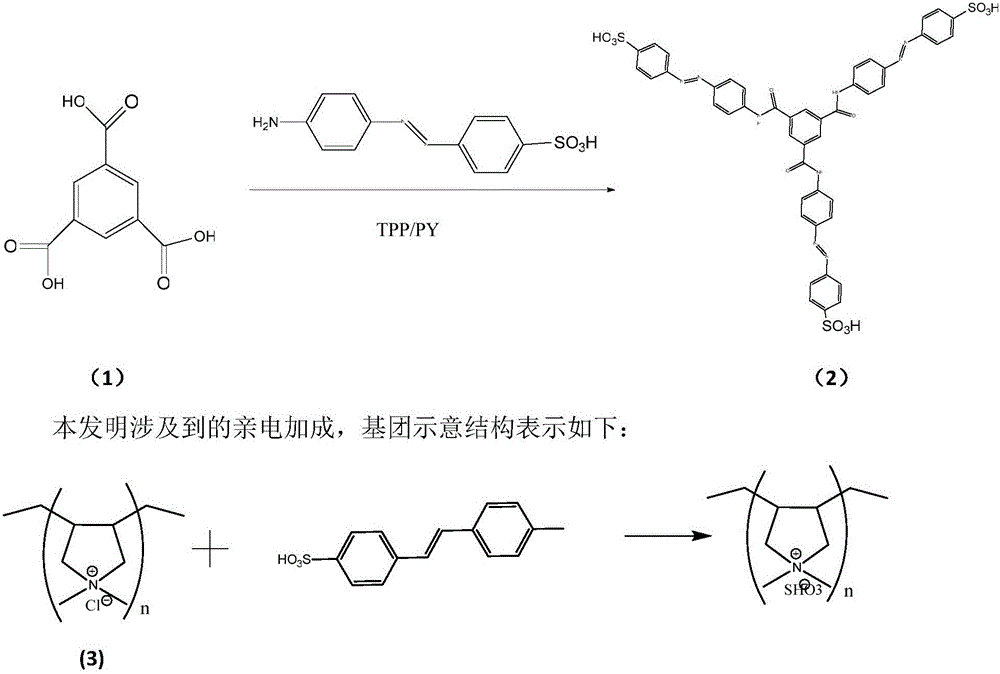
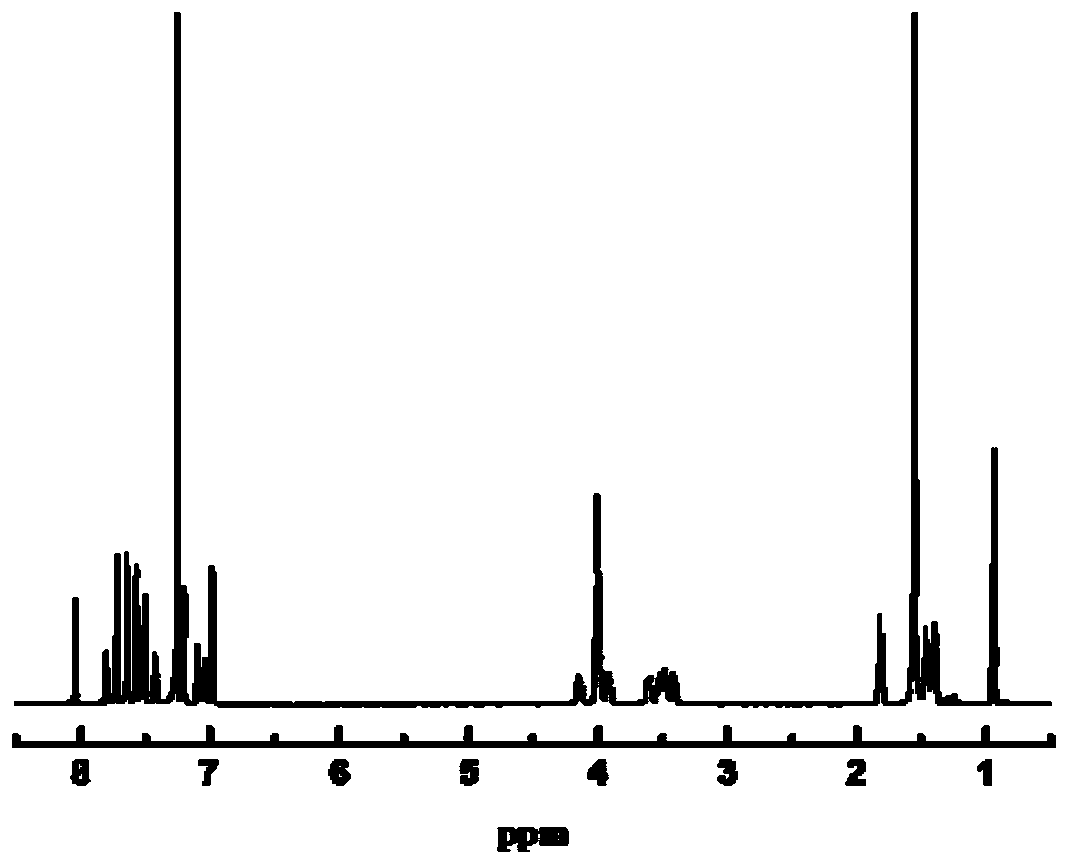
Tribranched azobenzene hybrid material with photoinduced deformation, and preparation method thereof
The invention relates to a tribranched azobenzene hybrid material with photoinduced deformation, and a preparation method thereof. A cross-linked structure formed through electrophilic addition of sulfonate radicals of tribranched azobenzene and ammonium ions in poly(diallyldimethylammonium chloride) is the deformed tribranched azobenzene / poly(diallyldimethylammonium chloride) hybrid material. The cis-trans isomerism of azo of a cross-linked polymer under lights influences the form of the polymer, so the prepared polymer bends and deforms under the lights, and becomes the tribranched azobenzene / poly(diallyldimethylammonium chloride) hybrid material with photoinduced deformation. The hybrid material is spin-coated to produce a film, the film has upward bending deformation under ultraviolet irradiation, and the bent film recovers to the original shape in 5-10 min after the ultraviolet lights are removed.
Owner:TIANJIN UNIV
Dinaphthalene azobenzene ring-type photosensitive chiral molecule and preparation method and application thereof
ActiveCN109810129ARealize reversible regulationGood compatibilityMaterial analysis by optical meansGroup 3/13 element organic compoundsN dimethylformamideBoronic acid
The invention discloses a dinaphthalene azobenzene ring-type photosensitive chiral molecule and a preparation method and application thereof. The preparation method comprises the steps that 6,6'dibromo-1,1'-bi-2-naphthol, potassium carbonate, potassium iodide and 2-(2-chloroethoxy)ethanol are dissolved in N,N-dimethylformamide, thus a midbody 1 is obtained, the midbody 1 and [4'-(pentyloxy)[1,1'-biphenyl]-4-yl]boronic acid are dissolved in 1,4-dioxane to react with a potassium carbonate aqueous solution and tetrakispalladium to obtain a midbody 2, the midbody 2 is dissolved through dichloromethane, and then triethylamine, 4-dimethylaminopyridine and paratoluensulfonyl chloride are added and react to obtain a midbody 3; and cesium carbonate is added into the midbody 3, 2,2'-dihydroxyazobenzene and dibenzo-18-crown ether-6 under the nitrogen atmosphere for reacting, and thus a target product T is obtained. The dinaphthalene azobenzene ring-type photosensitive chiral molecule is easy to synthesize and good in stability, the screw pitch of a cholesteric liquid crystal can be adjusted and controlled through photoinduced cis-trans isomerization, and good compatibility between the dinaphthalene azobenzene ring-type photosensitive chiral molecule and liquid crystal molecules is achieved.
Owner:HEFEI UNIV OF TECH
High-camptothecin compounds and use thereof as medicaments
InactiveCN102010418AHas antitumor activityOrganic active ingredientsOrganic chemistryDNA underwindingMedicinal chemistry
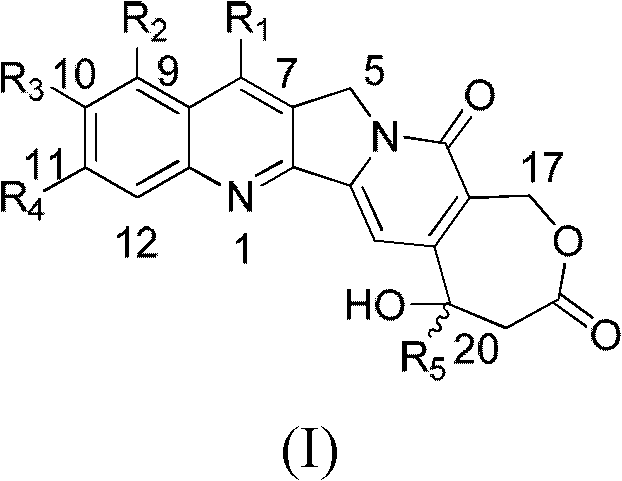
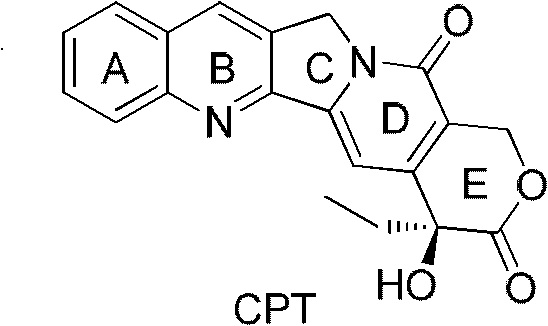
The invention relates to the technical field of medicaments, in particular to novel high-camptothecin compounds and use thereof as anti-tumor medicaments. The compounds have the structure shown in a general formula (I) and comprise optical isomers, racemes, trans-isomers and any mixture or pharmaceutical salts thereof in the forms. The compounds have the function of inhibiting the activity of topoisomerase and can be used for preparing the anti-tumor medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com