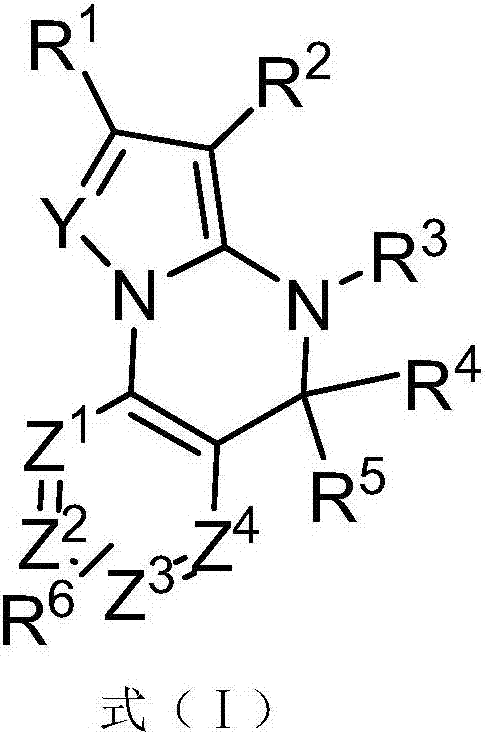
Fused heterocyclic compounds and application thereof
A technology of fused heterocyclic compounds, applied in the field of heterocyclic compounds, can solve the problems of restricting the use of pesticides, restricting the development of agriculture and forestry, and environmental issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
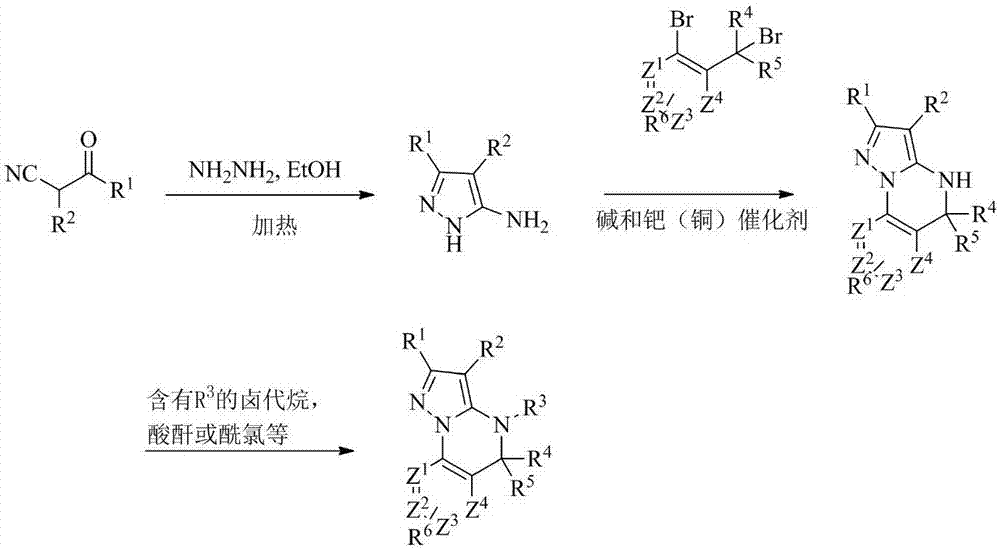
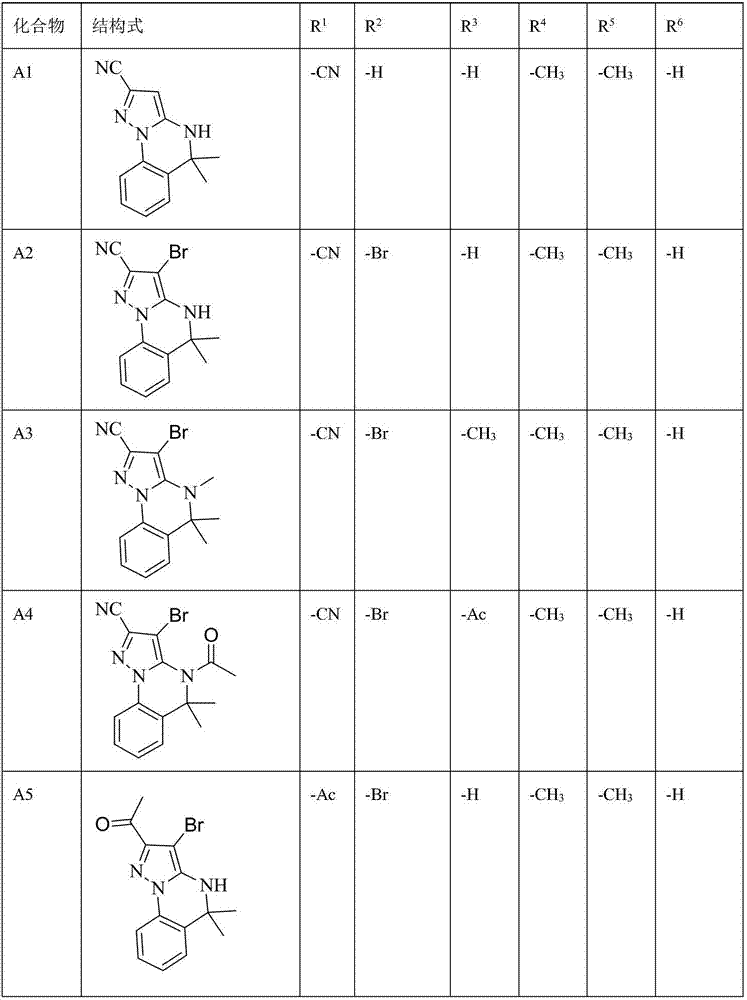
[0097] Example 1: Using method A to synthesize A1~A47 compounds
[0098] Method A
[0099]
[0100] For example, the synthesis of compound A1:
[0101] S1: 5-Amino-1H-pyrazole-3-cyano
[0102] Add ethyl 2,3-dicyanopropionate (1.0 mmol) to 20.0 ml of ethanol, stir vigorously, add hydrazine hydrate (2.0 mmol) to the reaction solution, heat to reflux, stop heating after 3 hours, and cool to room temperature. The mixture was concentrated in vacuo, the mixture was extracted with ethyl acetate and water, and the extracted ethyl acetate was concentrated in vacuo to give 5-amino-1H-pyrazole-3-cyano as a white solid, which was used without further purification in in the next step.
[0103] S2: 5,5-Dimethyl-4,5-dihydropyrazol[1,5-α]quinazoline-2-cyano
[0104] Under nitrogen protection, 5-amino-1H-pyrazole-3-cyano (1.0mmol), 1-bromo-2-(1-bromo-1-methylethyl)benzene (1.0mmol), cuprous iodide (0.2 mmol) and cesium carbonate (0.5 mmol) in DMF was stirred at 100 °C for 24 hours. C...
Embodiment 2
[0115] Example 2: Using Method B to Synthesize B1~B12 Compounds
[0116] Method B
[0117]
[0118] For example, the synthesis of compound B1:
[0119] S1:2-amino-4-(4-methoxybenzene)-1H-pyrrole-3-cyano
[0120] 2-Bromo-1-(4-methoxyphenyl)-acetyl (1.0mmol) was added in 15 ml of DMF, then sodium azide (10.0mmol) was added to the reaction solution, and the reaction mixture was stirred After 48 hours, the reaction mixture was filtered and the resulting filtrate was extracted several times with diethyl ether. Concentrate in vacuo, the residue is dissolved in methanol, under a hydrogen atmosphere, 10% palladium carbon (0.2 mmol) is added thereto, the reaction mixture is stirred for 24 hours, the mixture is filtered, the obtained filtrate is concentrated in vacuo, the residue is dissolved in tetrahydrofuran, Acetic anhydride (2.0mmol), triethylamine (2.0mmol) were added thereto, and after the reaction mixture was stirred for 5 hours, the mixture was concentrated in vacuo, an...
Embodiment 3
[0130] Example 3: Using method C to synthesize C1~C64 compounds
[0131] Method C
[0132]
[0133] For example, the synthesis of compound C1:
[0134] S1: (2-Amino-3-chloro-5-(trifluoromethyl)phenyl)boronic acid
[0135] Under nitrogen protection, 2-bromo-6-chloro-4-trifluoromethylaniline (1.0 mmol) was dissolved in 20 ml of dry tetrahydrofuran, cooled to 0 degrees Celsius, and n-butyl lithium (2.5 mmol, 1.6M n-hexane solution), stirred for 2 hours, slowly added trimethylchlorosilane (2.5mmol) dropwise to the reaction mixture, the reaction was naturally heated and stirred for 12 hours, concentrated in vacuo to remove the solvent, and obtained the target compound by distillation under reduced pressure (2-Amino-3-chloro-5-(trifluoromethyl)phenyl)boronic acid (yield: 87%).
[0136] S2: 2-chloro-6-(1,2-dichloro-3-buten-2-yl)-4-(trifluoromethyl)aniline
[0137] Under nitrogen protection, 3-bromo-3,4-dichloro-1-butene (1.0mmol), Pd(dppf)Cl 2 (0.15mmol), sodium carbonate (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com