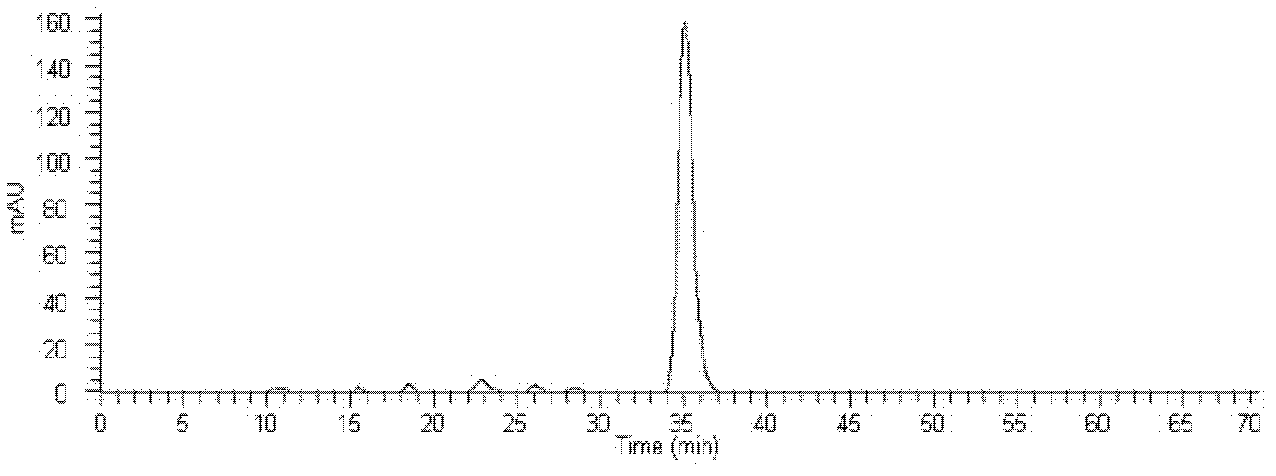
HPLC-MS detection method of xanthophylls cis-trans-isomers in xanthophylls products
A technology of cis-trans isomerization and detection method, which is applied in the field of liquid chromatography analysis, can solve the problems of poor separation effect of lutein isomers, relatively expensive prices of acetonitrile and methyl tert-butyl ether, and achieve separation Good effect, extended retention time, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
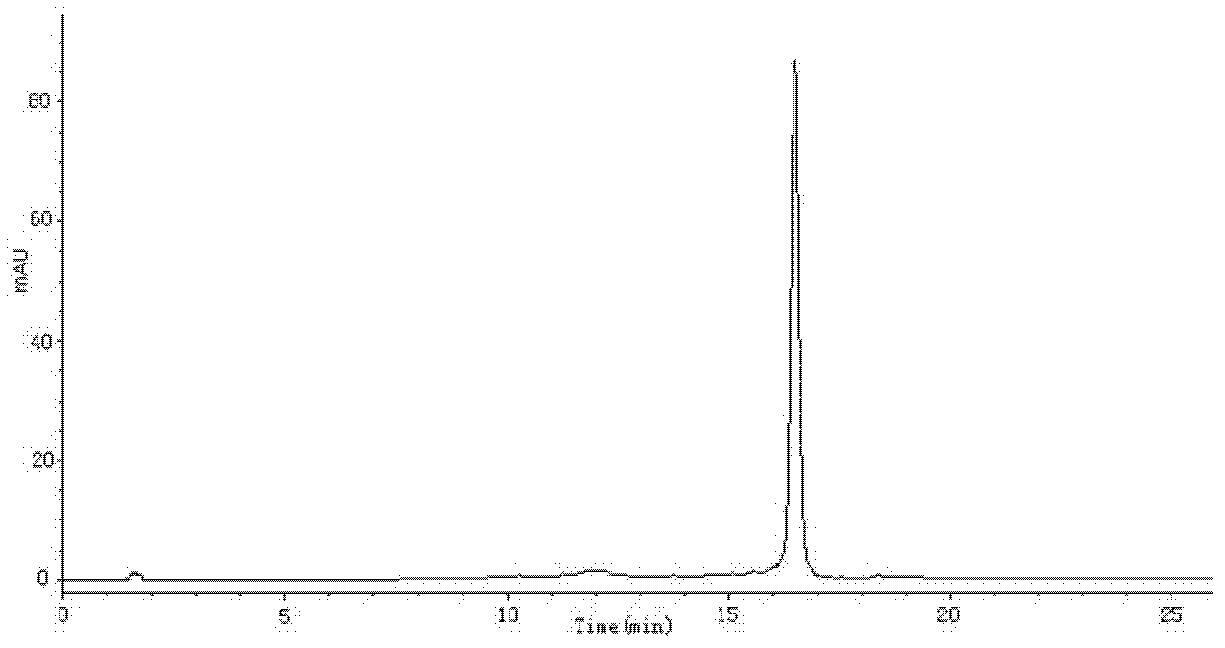
[0048] Weigh 0.01 g of the content of lutein soft capsules into a conical flask, add 30 mL of extractant (hexane-acetone-ethanol-toluene), plug the stopper and fully rotate and shake. Use a pipette to add 2mL of 40% KOH methanol solution to the Erlenmeyer flask, rotate and shake for 1min, seal overnight (12h), transfer the sample solution to a separatory funnel, wash with 10% sodium sulfate, and wash several times to remove the lye . Take the supernatant, concentrate to dryness under reduced pressure, dilute to 10 mL with methanol, and filter the sample liquid through a 0.45 μm microporous membrane for later use. HPLC-MS detection of lutein isomers in soft capsules, in which the content of all-trans lutein is 3.68mg / g, the content of 9-cis-lutein is 0.056mg / g, and the content of 9'-cis-lutein 0.06mg / g, 13-cis-lutein content 0.12mg / g and 13'-cis-lutein content 0.128mg / g.
[0049] C 30 -HPLC analysis conditions: chromatographic column is YMC Carotenoid C 30 (4.6mm×250mm, 5μm...
Embodiment 2
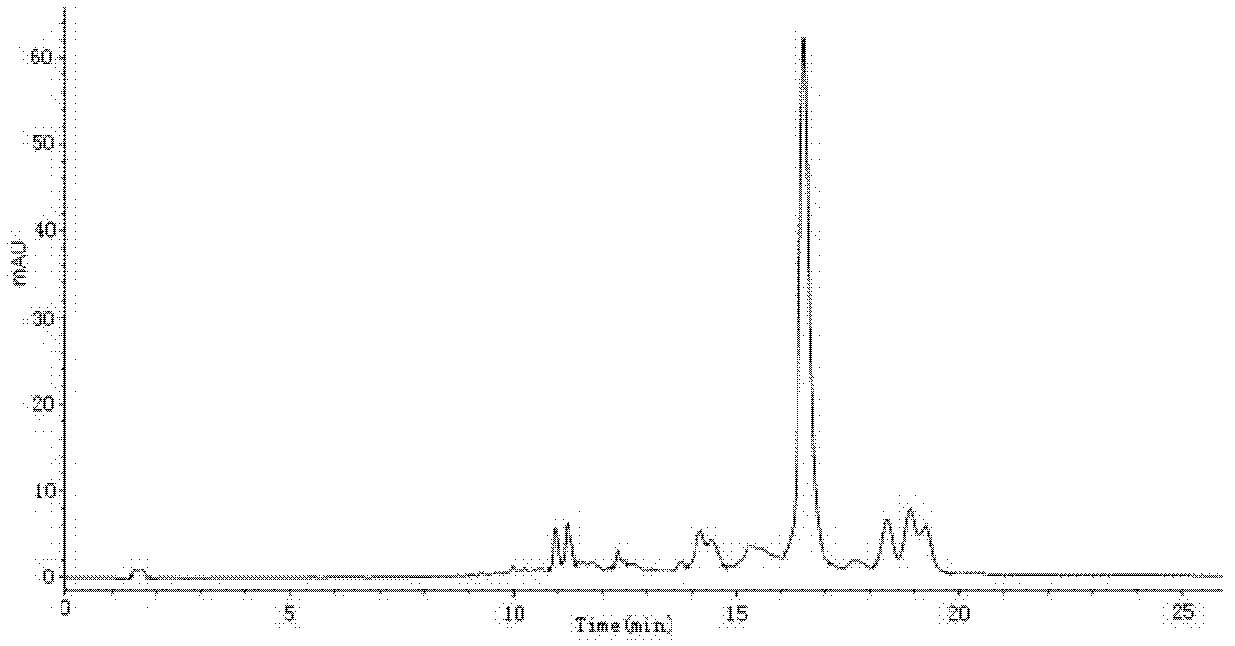
[0051] Powder the lutein tablet, weigh 0.01g of the powder into a conical flask, add 30mL extractant (hexane-acetone-ethanol-toluene), plug the stopper and fully rotate and shake. Use a pipette to add 2mL of 40% KOH methanol solution to the Erlenmeyer flask, rotate and shake for 1min, seal overnight (12h), transfer the sample solution to a separatory funnel, wash with 10% sodium sulfate, and wash several times to remove the lye . Take the supernatant, concentrate to dryness under reduced pressure, dilute to 10 mL with methanol, and filter the sample liquid through a 0.45 μm microporous membrane for later use. HPLC-MS detection of lutein isomers in the tablet, in which the content of all-trans lutein is 13.76mg / g, the content of 9-cis-lutein is 0.35mg / g, and the content of 9'-cis-lutein 0.37mg / g, 13-cis-lutein content 0.77mg / g and 13'-cis-lutein content 0.82mg / g.
[0052] C 30 -HPLC analysis conditions: chromatographic column is YMC Carotenoid C 30 (4.6mm×250mm, 5μm), mobil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com