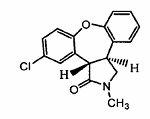
New process for synthesis of asenapine
A new process and compound technology, applied in the field of arsenic, can solve the problems of low total reaction yield, difficult monitoring of the reaction process, difficult to obtain, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of compound 11
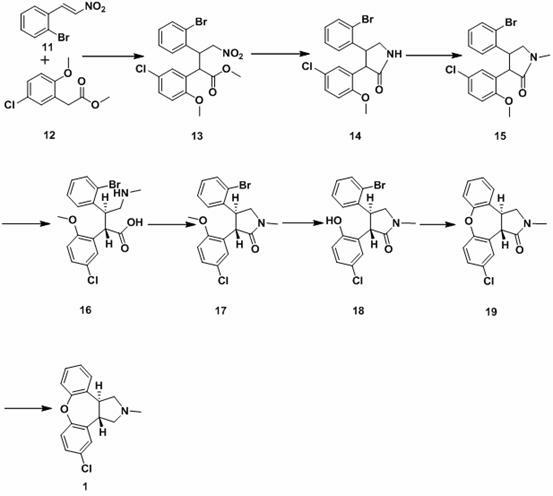
[0038] Reflux 68g of o-bromobenzaldehyde, 200ml of nitromethane and 40g of ammonium acetate in 50ml of glacial acetic acid for 2 hours. After the reaction, add 1300ml of water to the system, stir well and filter, and the obtained yellow crude product is dissolved in ethanol Recrystallized to obtain 78 g of 2-bromo-β-nitrostyrene, namely compound 11, with a yield of 87.2%.
Embodiment 2
[0039] Embodiment 2: the preparation of compound 13
[0040] Dissolve 38.6g of dry isopropanol in 150ml of dry tetrahydrofuran (THF), cool the system to below -60 degrees Celsius, and add 192ml of n-butyl lithium in n-hexane dropwise while stirring. After stirring the reaction for 30 minutes, a solution of 94.5 g of methyl 2-methoxy-5-chlorophenylacetate in 300 ml of dry THF was added dropwise. After stirring for a further 15 minutes, a solution of 64.4 g of 2-methoxy-β-nitrostyrene (11) in 600 ml of dry THF was added dropwise with stirring, keeping the temperature below 50°C. Stir the reaction for 30 minutes after the addition, add a small amount of water to quench the reaction, evaporate most of the THF under reduced pressure, add an appropriate amount of 6N hydrochloric acid solution to the residue to acidify the solution, extract with dichloromethane two to three times, combine the organic layers, and add saturated salt Washed with water, dried over anhydrous magnesium su...
Embodiment 3
[0041] Embodiment 3: the preparation of compound 14
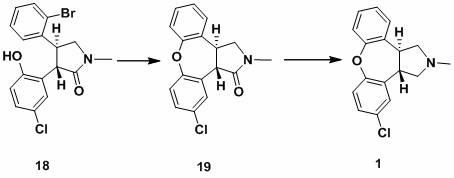
[0042] Dissolve the oily compound 13 obtained in the previous step reaction in 1000ml of ethanol, add 10g of 10% Pd / C, hydrogenation reaction overnight, filter to remove the catalyst after the reaction, concentrate the filtrate under reduced pressure, add an appropriate amount of diethyl ether to obtain off-white solid, ethanol Recrystallization gave 103 g of the refined product of amide compound 14 (a mixture of cis and trans isomers), and the two-step yield was 76.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com