Patents
Literature
134results about How to "Starting materials are readily available" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation of 2-bromo-4,5-dimethoxy benzenepropanenitrile
InactiveCN101407474AHigh yieldStarting materials are readily availableCarboxylic acid nitrile preparationOrganic compound preparationFiltrationBenzaldehyde
The invention relates to a method for preparing 2-bromine-4, 5-dimethoxy benzene propane nitrile, which consists of steps: 3-4-dimethoxy benzaldehyde and bromine are dissolved in acetic acid to be reacted; when solid is precipitated, 2-bromine-4, 5-dimethoxy benzaldehyde crystal is obtained through suction filtration; the crystal and acetonitrile are dissolved in organic solvent; after a catalytic reaction, yellow solid is precipitated, cooled, extracted and dried to get 2-bromine-4, 5-dimethoxy cinnamonitrile solid which is then dissolved in methanol with reducing agent and is reduced to obtain 2-bromine-4, 5-dimethoxy benzene propane nitrile after a reflux reaction; or the obtained 2-bromine-4, 5-dimethoxy cinnamonitrile is reduced with hydrogen under the catalysis of palladium-charcoal to get 2-bromine-4, 5-dimethoxy benzene propane nitrile. The 2-bromine-4, 5-dimethoxy benzene propane nitrile prepared by the invention has high yield coefficient; original raw materials of the preparation method are easy to get; the price is low; the reaction operation is simple; the reaction route is short; and the method is easy for industrialization production.
Owner:DONGHUA UNIV
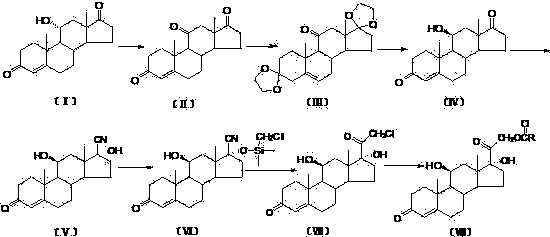
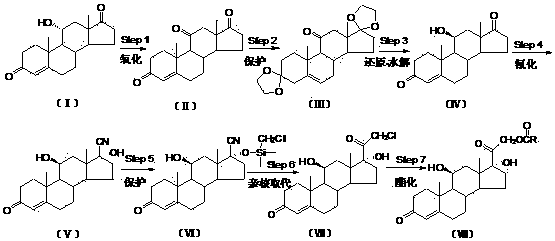
Production process capable of industrially synthesizing dydrogesterone
The invention discloses a production process capable of industrially synthesizing dydrogesterone. Easily available progesterone is used as a raw material, and dydrogesterone is prepared through the steps of carbonyl protection, bromination, debromination, photochemical ring-opening reaction, photochemical ring-closing reaction, deprotection and double bond isomerization. The production process hasthe advantages of easily available initial raw materials, and easiness in implementation of each step and higher yield; the production process is simple and convenient to operate, is green and environment-friendly, and can be easily amplified to industrial production.
Owner:GUANGXI NORMAL UNIV
Method for synthesizing 2,3,4-trifluoro phenyl formic acid
InactiveCN1358708AStarting materials are readily availableLow priceOrganic compound preparationCarboxylic compound preparationGrignard reagentSynthesis methods
The present invention relates to two synthesis methods for preparing 2,3,4-trifluorobenzoic acid, in which 2,3,4-trifluoroaniline is used as raw material, the diazotization of amino group and nitrile grouping actino are used to obtain 2,3,4-trifluorobenzonitrile, processed with acid hydrolysis to obtain the invented product, in another mether, 2,3,4-trifluoroaniline processed by diazotization of amino group and bromization to obtain 1-bromo-2,3,4-trifluorobenzene, then reacted with metal magnesium to obtain Grignard reagent, and reacted with carbon dioxide and processed by acid hydrolysis so as to synthesize the invented product.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI +2
Preparation method and detection method for edaravone dimer and tautomer thereof
ActiveCN102180833ASimple and fast operationStarting materials are readily availableOrganic chemistryComponent separationSolventHigh-performance liquid chromatography
The invention belongs to the pharmaceutical field, and specifically relates to a preparation method and a detection method for edaravone dimers and tautomers thereof. The preparation method is characterized in that edaravone is dissolved in a solvent; a catalyst is added; the mixture is heated and filtered to obtain an insoluble substance; the substance is washed and dried to obtain a product. The detection method adopts a high performance liquid chromatography method. The preparation method of the invention has convenient operation and easily available initial raw materials, and the detection method is applicable to the detection of edaravone dimers in edaravone raw materials and preparation samples.
Owner:NANJING CHANGAO PHARMA SCI & TECH CO LTD
Preparation method of hydrocortisone acetate or analogue thereof
Owner:HUNAN NORCHEM PHARMACEUTICAL CO LTD
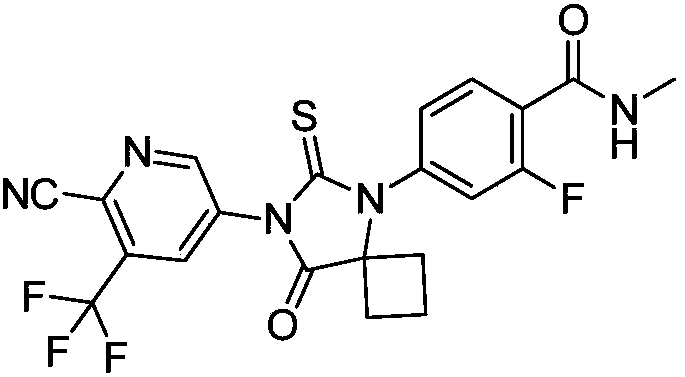
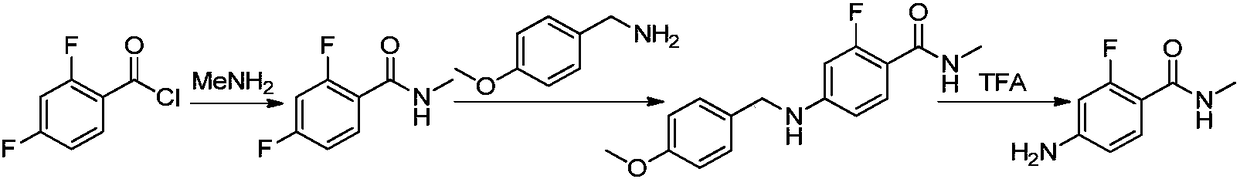
Method for synthesizing apalutamide and intermediate thereof
ActiveCN108383749AHigh crystallinityImprove stabilityOrganic compound preparationCarboxylic acid amides preparationMethyl groupHydrochloride
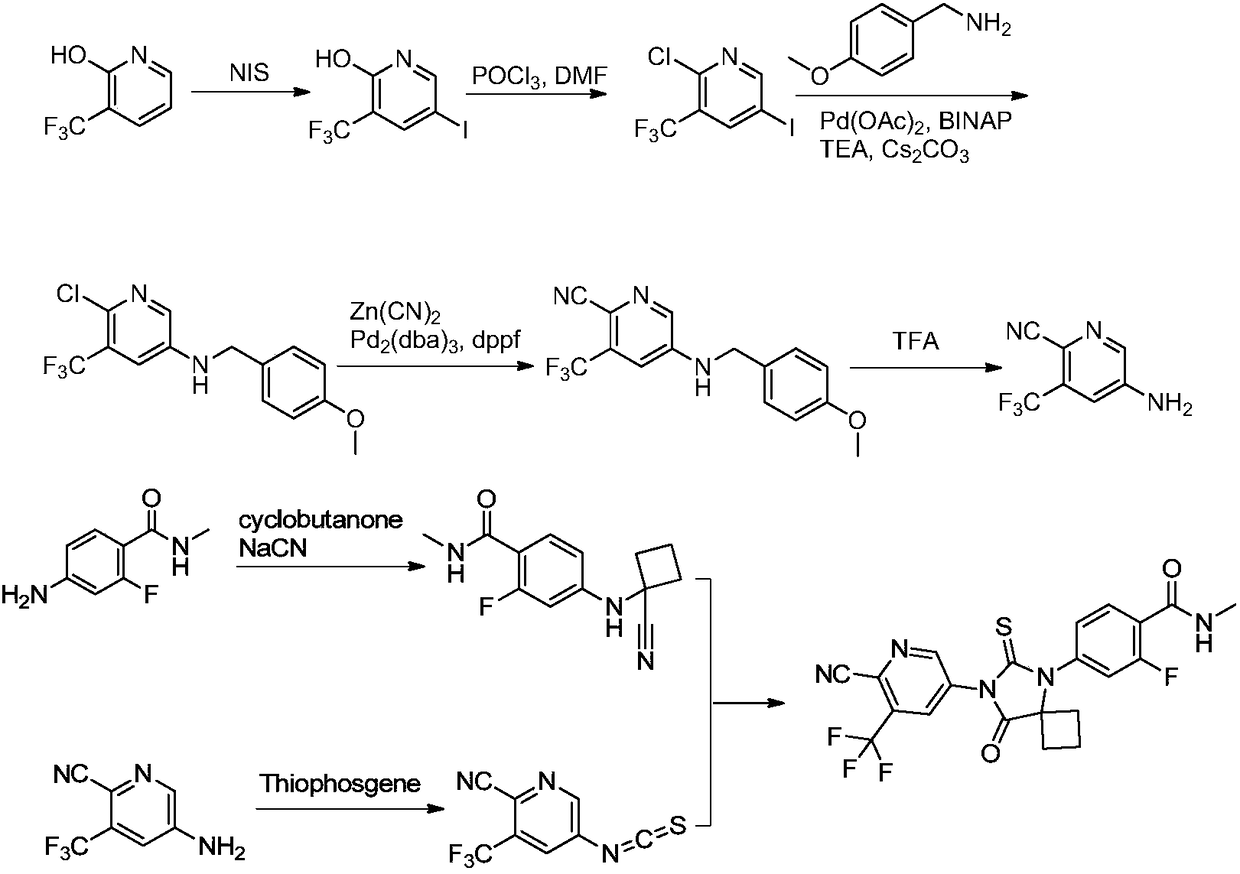
The invention discloses a method for synthesizing apalutamide. The method includes carrying out condensation on N-methyl-2-fluorine-4-halogenated-benzamide compounds 1 and amino-1-cyclobutanecarboxylic acid hydrochloride 2 by means of Ullmann reaction to obtain to obtain intermediate compounds 3 and esterifying the intermediate compounds 3 to obtain intermediate compounds 4; carrying out cyclization by means of reaction on the intermediate compounds 4 and thiocyanide to obtain compounds 5; carrying out condensation by means of coupling the compounds 5 to obtain the apalutamide. The N-methyl-2-fluorine-4-halogenated-benzamide compounds 1 and the amino-1-cyclobutanecarboxylic acid hydrochloride 2 are used as starting materials. A path is shown, an R in the path represents alkyl, includes butis not limited to methyl or ethyl. The method has the advantages that route steps can be shortened to a great extent, the route efficiency can be improved, precious metal catalysts are omitted, and accordingly the process cost can be lowered; byproduct generation can be reduced, and accordingly the method is favorable for improving the purity of ultimate products.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
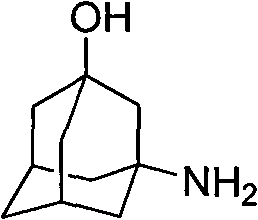
Method for preparing 3-amino-1-adamantane alcohol
InactiveCN101798270AReduce dosageReduce processing costsOrganic compound preparationAmino-hyroxy compound preparationWater bathsIce water
The invention relates to a method for preparing 3-amino-1-adamantane alcohol. The method comprises the following steps of: adding amantadine hydrochloride into a nitrating agent in batches, performing reaction for 1 to 2 hours in an ice-water bath and performing reaction for 1 to 30 hours at room temperature to obtain yellowish liquid; pouring the yellowish liquid into ice, continuously reacting for 0.5 to 2 hours with stirring to obtain blue-green liquid; and adding solid base into solution obtained by the step 2 with stirring, keeping temperature below 80 DEG C, regulating pH to be between 10 and 12, performing reaction for 30 minutes with stirring, leaching, extracting reaction liquid by using dichloromethane, drying the obtained product with anhydrous sodium sulfate, steaming off the dichloromethane and performing recrystallization by using ethyl acetate to obtain white solid. The preparation method has the advantages of readily available starting raw materials, simple reaction operation, short route, environmental friendliness, easy industrial production and good application prospect, and also reduces cost for the synthesis of Vildagliptin serving as a medicament for treatingdiabetes; and the yield of products reaches 75 percent.
Owner:DONGHUA UNIV
Preparation method of optical active medicine intermediate
ActiveCN103570601AStarting materials are cheapStarting materials are readily availableOrganic chemistryMedicinal chemistryHydrochloride
The invention relates to a preparation method of an optical active compound, represented by formula I, or a hydrochloride of the optical active compound by taking a compound with optical activity as a starting material. Raw materials of the preparation method are cheap and easily available; no splitting is needed; the whole technological operation is simple and convenient; cost is low; pollution on environment is less; and the preparation method is suitable for industrialized production. In the formula I, n is 1 or 2 or 3.
Owner:PORTON FINE CHEM
Mesosulfuron preparation method
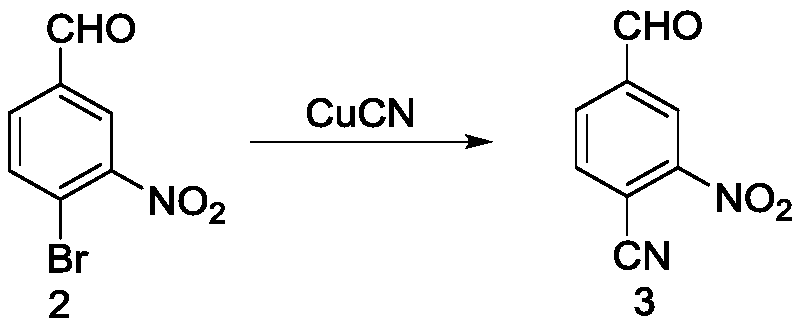
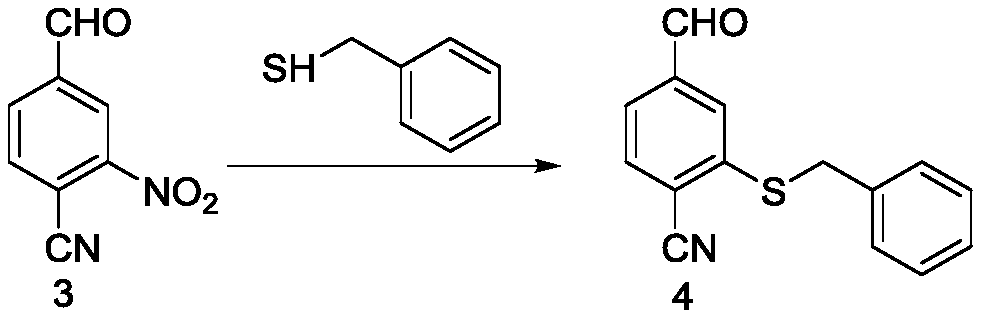
The invention relates to a mesosulfuron preparation method which includes the steps: taking p-bromo benzaldehyde as a starting material, and performing nitration reaction; performing reaction of a reactant and cuprous cyanide, and substituting bromine with cyan; performing nucleophilic substitution reaction on the reactant and benzyl mercaptane, performing reaction on the reactant and hydroxylamine hydrochloride to obtain an oxime intermediate, and reducing oxime by zinc powder; acylating methyl sulfone chloride, hydrolyzing the cyan by concentrated alkaline to obtain carboxylic acid, esterifying the carboxylic acid, chloridizing the esterified carboxylic acid by chlorine, and ammonifying chloridized carboxylic acid by ammonia to obtain 2-methoxycarbonyl-5-methanesulfonyl aminomethyl benzene sulfonamide serving as an intermediate; performing coupling to obtain mesosulfuron serving as a target product. The preparation method is simple to operate, environmentally friendly and high in yield and serves as a novel good mesosulfuron synthesis method.
Owner:YANGZHOU UNIV +1
Method for preparing voriconazole and intermediate thereof
ActiveCN102807563AStarting materials are readily availableMild reaction conditionsOrganic chemistryHalogenOrganic reaction
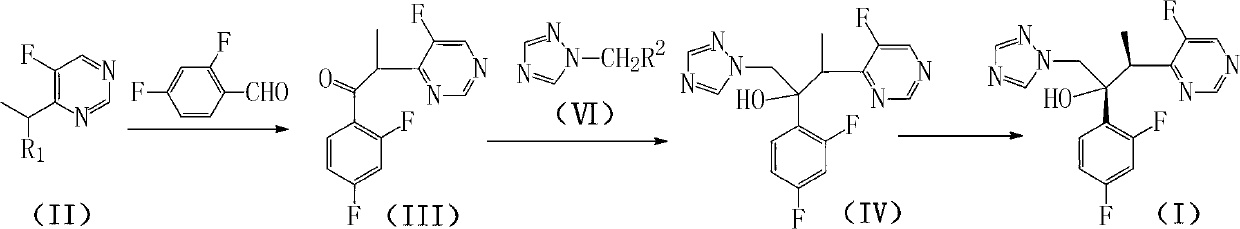
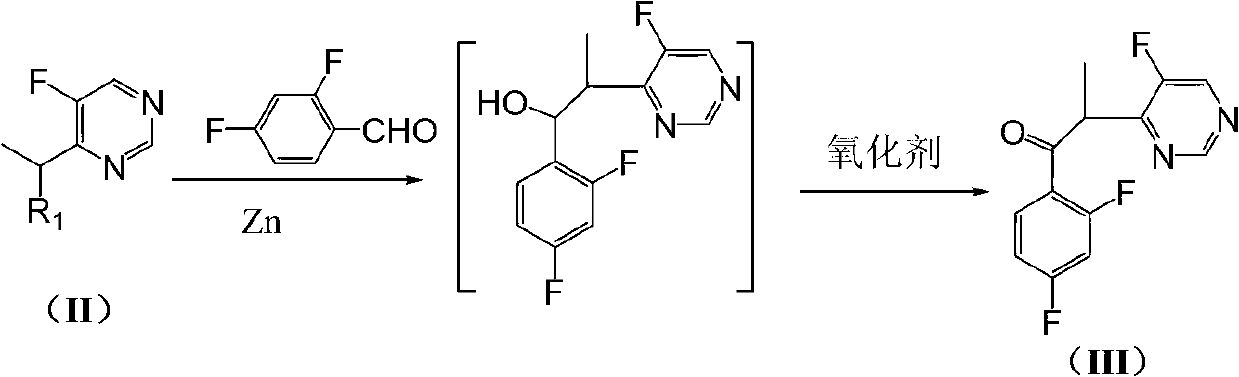
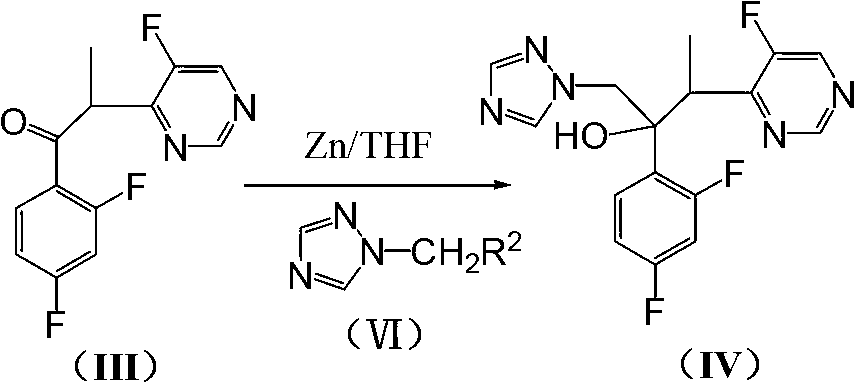
The invention discloses a method for preparing voriconazole and intermediate thereof. The method comprises the following steps of: enabling the compound 4-(1-halogenated ethyl)-5-fluoropyrimidine in the formula (II) to react with 2,4-difluorobenzaldehyde to generate the compound alpha-(5-fluoropyrimidine-4-yl)-2,4-difluoroacetone in the formula (III); then reacting with the compound 1-halogen methyl-1,2,4-triazole in the formula (VI) to generate the compound 1-(1,2,4-triazole-1-yl)-2-(2,4-difluorophenyl)-3-(5-fluoropyrimidine-4-yl)-2-butanol in the formula (IV); and finally resolving to obtain the voriconazole, shown as the accompanying drawing, wherein R1 and R2 are respectively halogen. The method for preparing voriconazole is easy in raw material obtaining, moderate in reaction conditions, and short in steps; each reaction is the mature organic reaction; and the method is widely applied in industry, and has certain industrial production conditions.
Owner:NANJING HUAWE MEDICINE TECH DEV
Preparation method of 3-(2-methylindolyl-3-)pyrryl-2,5-dione
The invention relates to a preparation method of 3-(2-methylindolyl-3-)pyrryl-2,5-dione, which comprises the following steps: (1) adding 2-methylindole, maleimide and Lewis acid in a mol ratio of 1.0:(1.0-1.5):(0.2-1.0) into reaction solvent, and reacting under reflux at room temperature to 100 DEG C for 5-24 hours, thereby obtaining a yellow white solution; and (2) evaporating to remove the solvent, adding water, stirring, carrying out vacuum filtration, and recrystallizing the obtained solid with ethanol to obtain the 3-(2-methylindolyl-3-)pyrryl-2,5-dione. In the process of preparing the 3-(2-methylindolyl-3-)pyrryl-2,5-dione, by using boron trifluoride and other Lewis acid as a catalyst, the reaction time is shortened, and the treatment of three wastes is lowered. The method has the advantages of higher yield, accessible raw materials, low cost, simple reaction operation and short reaction route, can easily implement industrial production, and has wide favorable application prospects.
Owner:DONGHUA UNIV
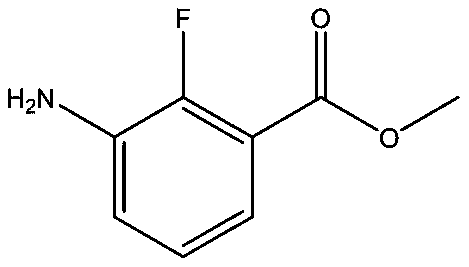
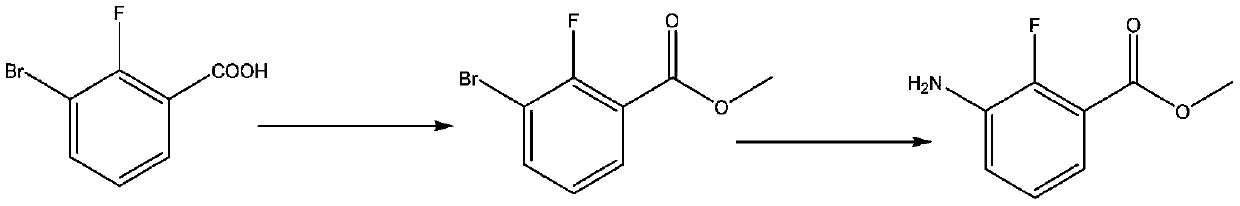
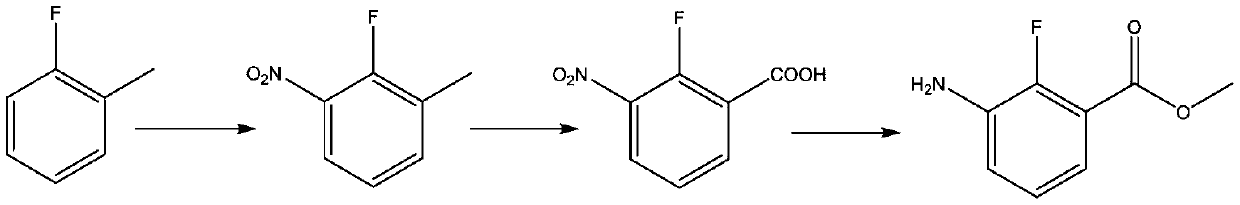
Synthesis method of anti-cancer drug intermediate methyl 2-fluoro-3-aminobenzoate
ActiveCN111320548AStarting materials are readily availableResponse Continuity IncreasedOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidNitration
The invention discloses a synthetic method of 2-fluoro-3-methyl aminobenzoate, and belongs to the technical field of synthesis of medical intermediates. According to the method, 2, 6-dichlorobenzoic acid is taken as a raw material, 2, 6-dichloro-3-nitrobenzoic acid is obtained through nitration reaction and high selectivity, then the 2, 6-dichloro-3-nitrobenzoic acid reacts with methanol under anacidic condition to form ester, then 2-fluoro-3-nitro-6-methyl chlorobenzoate is obtained through selective fluorination, and finally 2-fluoro-3-methyl aminobenzoate is obtained through catalytic hydrogenation. By adopting the process route provided by the invention, the initial raw materials are easy to obtain, common unit operation in fine chemical engineering is adopted in the process, the reaction continuity is increased, industrial operation is facilitated, and a basis is provided for large-scale application of downstream medicines.
Owner:浦拉司科技(上海)有限责任公司
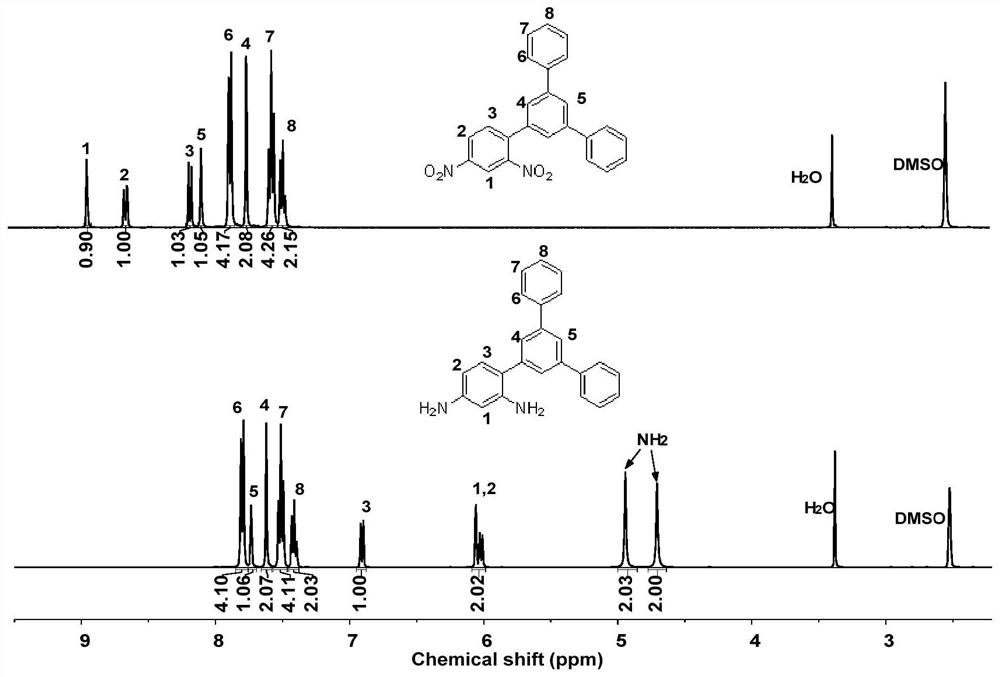
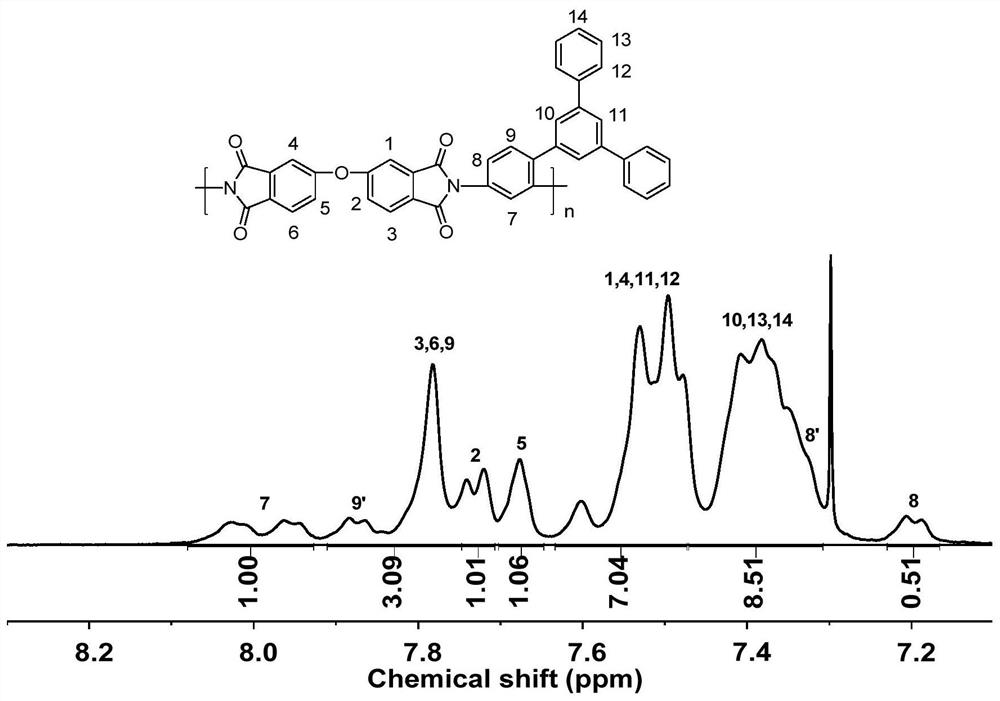
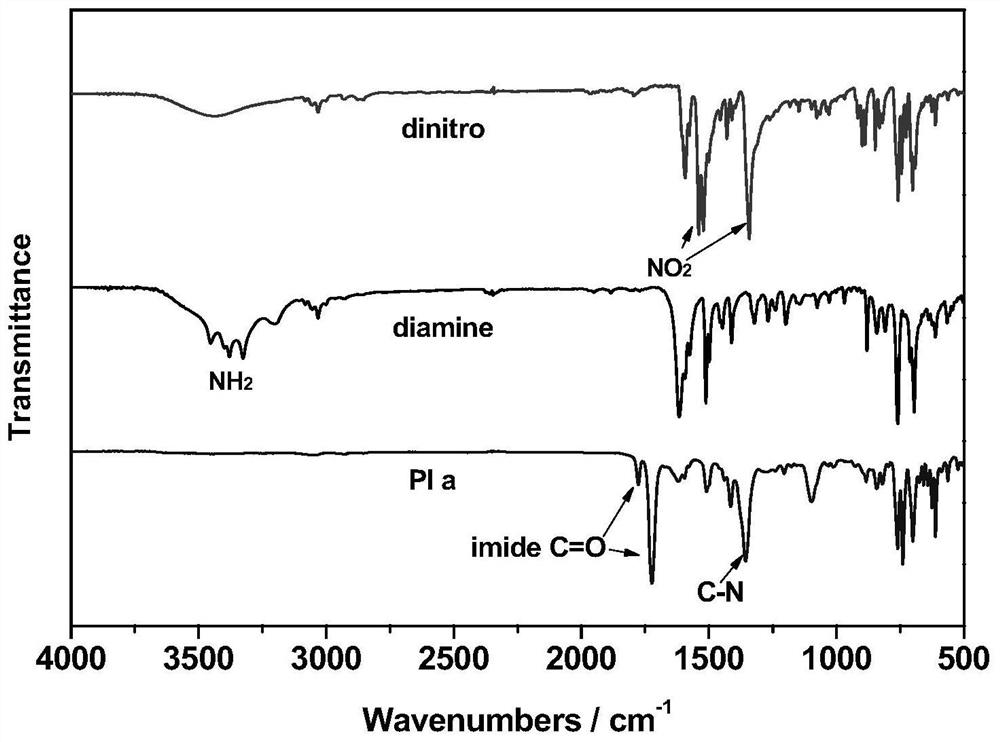
Asymmetric aromatic diamine monomer containing terphenyl large substituent side groups and polyimidecontaining terphenyl large substituent side groups
PendingCN113277950AThe synthetic route is simpleEasy to purify and separateOrganic compound preparationAmino compound preparationBromobenzeneBoronic acid
The invention belongs to the field of high polymer materials, and relates to an asymmetric aromatic diamine monomer containing terphenyl large substituent side groups and polyimide containing terphenyl large substituent side groups. 2, 4-dinitrobromobenzene reacts with 3, 5-diphenyl phenylboronic acid according to a molar ratio under an alkaline condition to obtain an intermediate compound, and the intermediate, the dinitro compound is reduced through a reduction reaction to obtain the asymmetric aromatic diamine monomer 3, 5-(diphenyl) phenyl-2, 4-diaminobenzene containing the terphenyl large substituted structure. The diamine monomer and a commercial aromatic dianhydride monomer which are equal in substance amount are added into an organic solvent, the solution is stirred to react under the action of a catalyst, and settling, washing and drying are performed to obtain the fibrous asymmetric structure polyimide containing the terphenyl large substituted side groups. The solubility of the polyimide in a specific solvent can reach more than 30wt%, the polyimide has excellent film-forming property, and a prepared polymer film has potential application value in the field of microelectronics.
Owner:CHANGZHOU UNIV
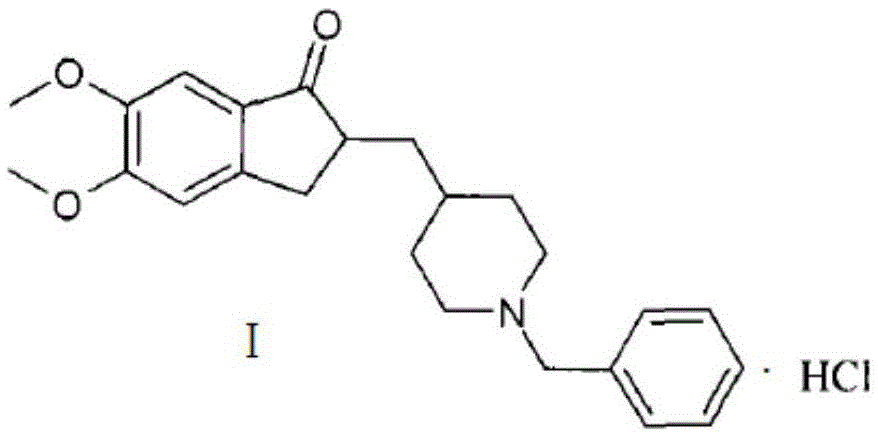
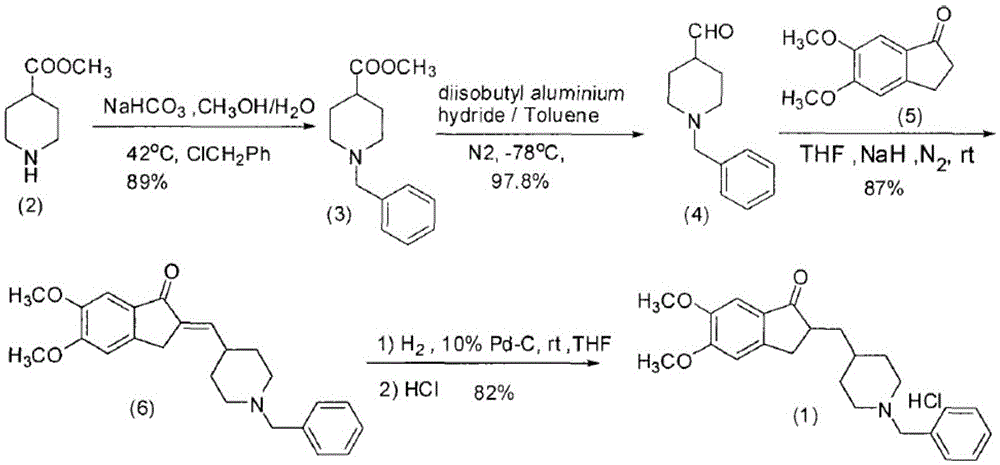
Preparation method of donepezil hydrochloride
ActiveCN105418488AReduce purificationStarting materials are readily availableOrganic chemistryKetoneIonic liquid
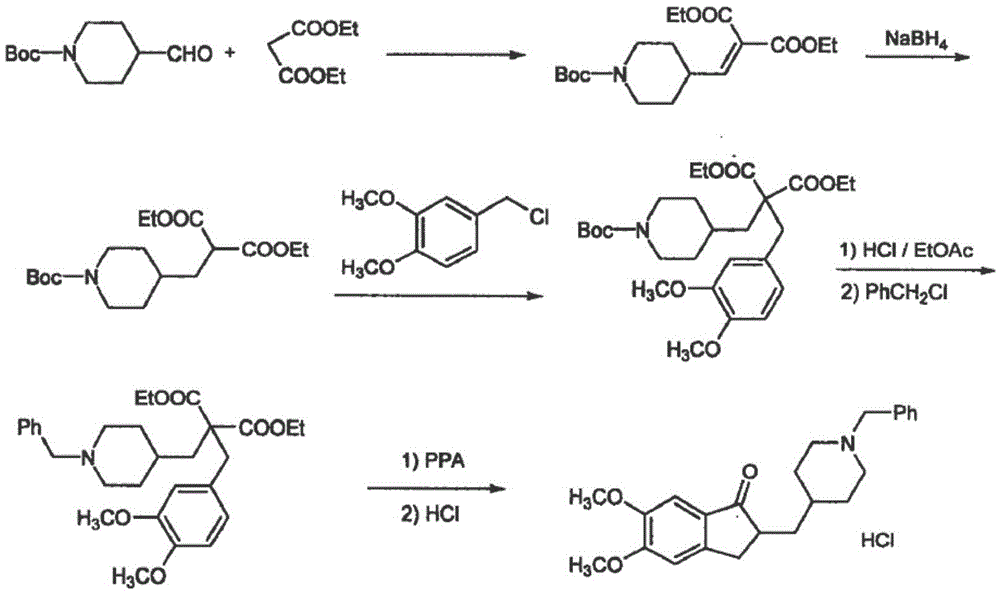
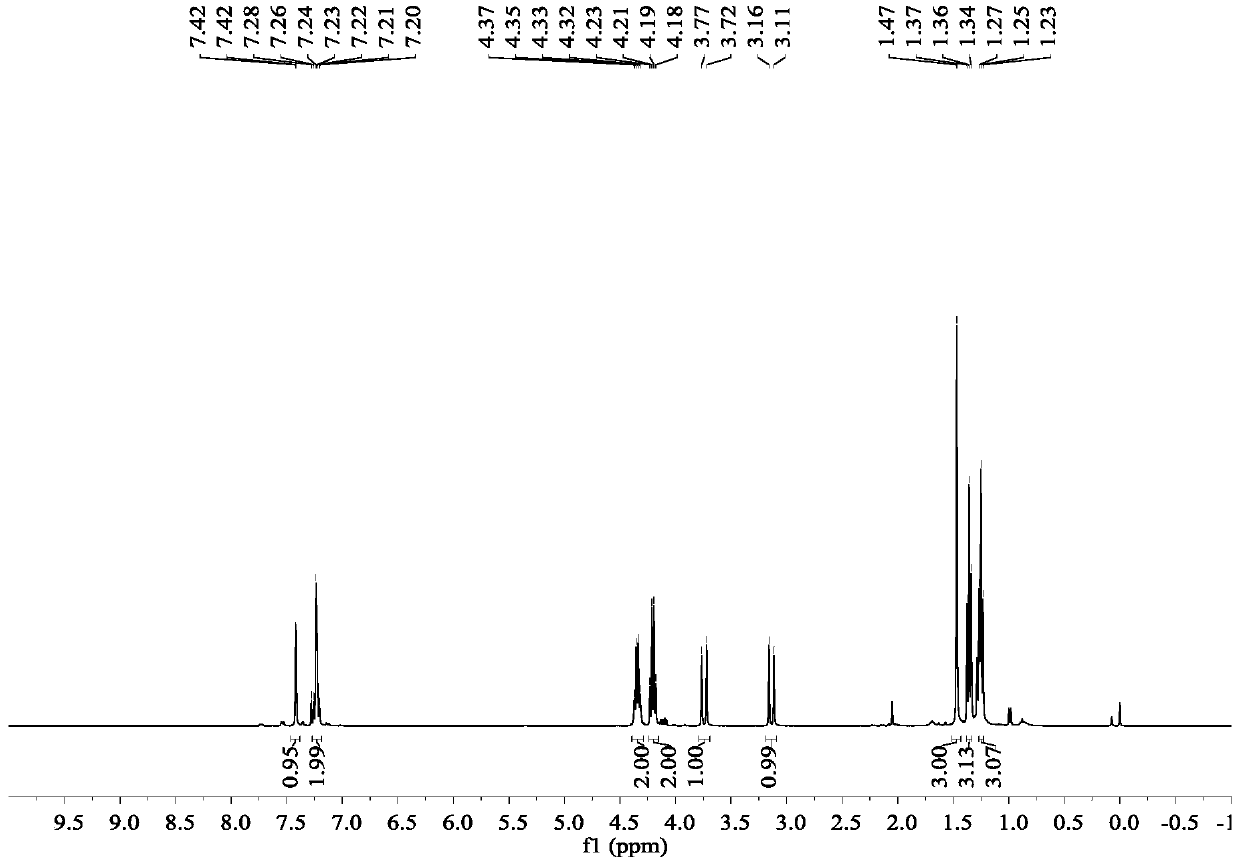
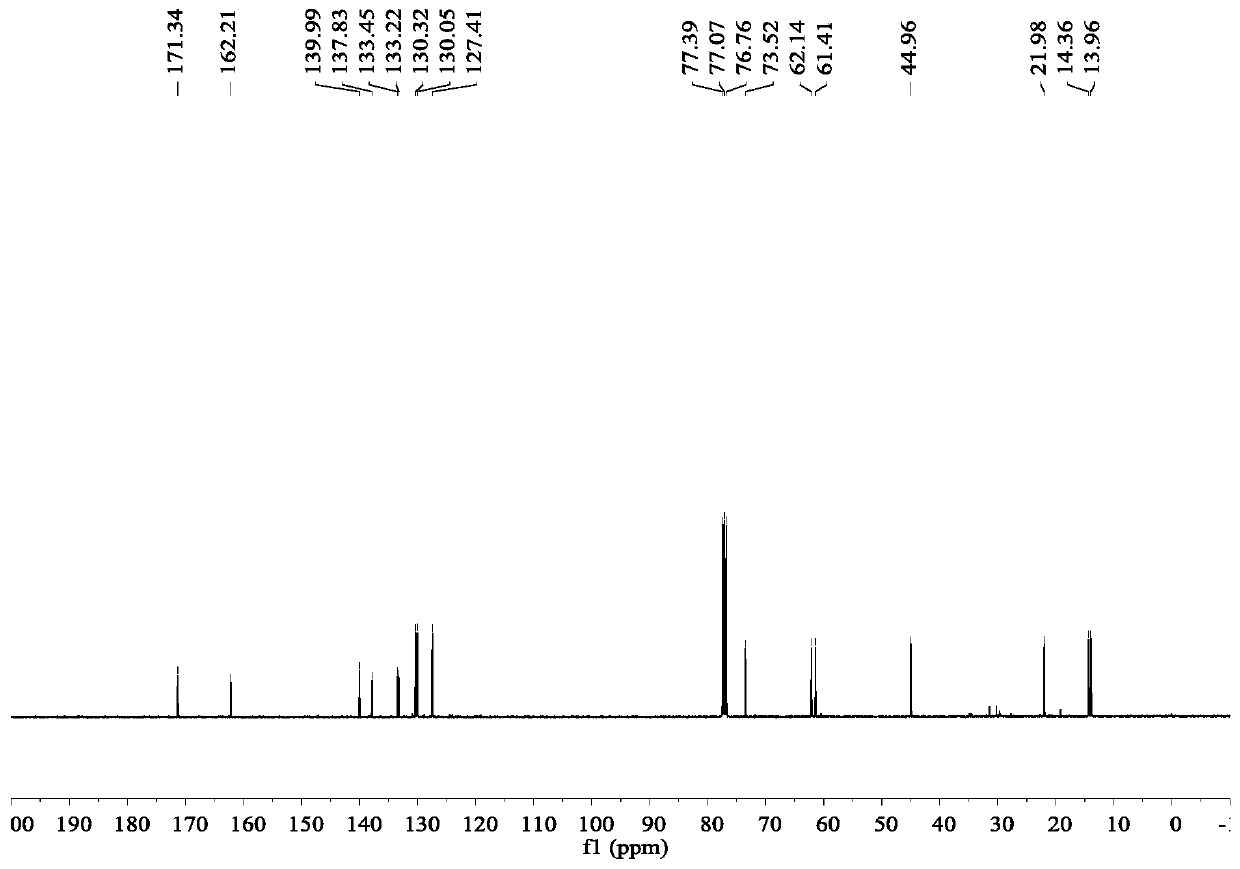
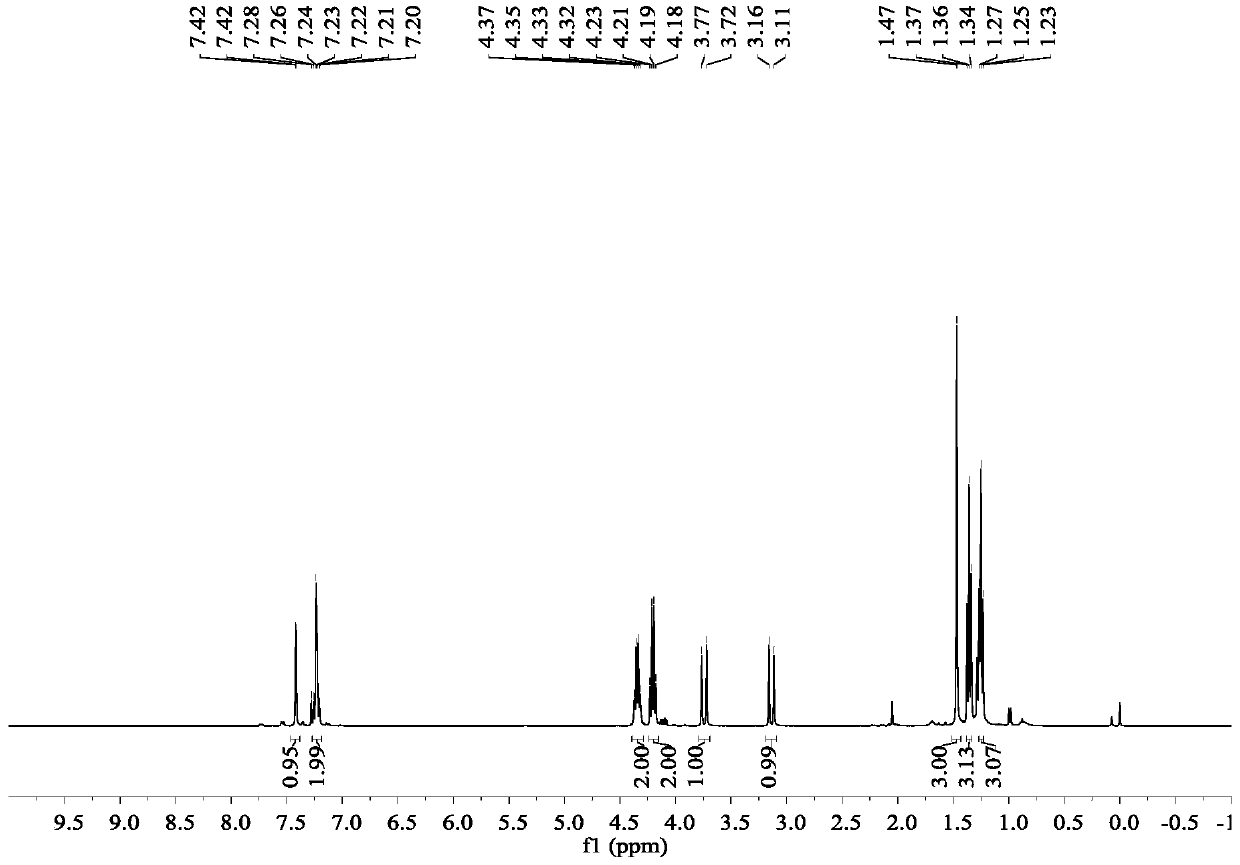
The invention discloses a preparation method of donepezil hydrochloride. The preparation method comprises the steps that 3-chlorine-1-(3, 4-dimethoxy phenyl) propane-1-ketone (II) is made to react with N-benzyl-4-formyl-piperidine (III) under the condition of a lewis acid ionic liquid catalyst, and 1-benzyl-4-(5, 6dimethoxy-1-indanone-2-methylene)-piperidine (IV) is obtained through a one-pot method, and then the donepezil hydrochloride (I) is obtained through reduction and salt formation. The preparation method of the donepezil hydrochloride has the advantages of being simple in process, low in production cost, environmentally friendly and suitable for industrialized production.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Synthesis method of mefenpyr-diethyl
ActiveCN110003107AStarting materials are readily availableShort reaction timeOrganic chemistryHydrazoneSynthesis methods
The invention relates to a synthesis method of mefenpyr-diethyl. The method comprises the steps of carrying out a reaction among 2,4-dichlorophenylhydrazine hydrochloride, ethyl glyoxalate and alkaliin a solvent, and carrying out a [3+2] cycloaddition reaction between obtained 2,4-dichlorophenylhydrazine ethyl glyoxylate hydrazone and ethyl methacrylate in a solvent under the function of a catalyst and an oxidant. According to the synthesis method, the starting raw materials are easy to obtain, the reaction time is shortened, the reaction path is short, the cost is low, the operation is simple, and three-waste treatment is lowered; under the same condition, the yield is higher, and industrial production is facilitated.
Owner:DONGHUA UNIV
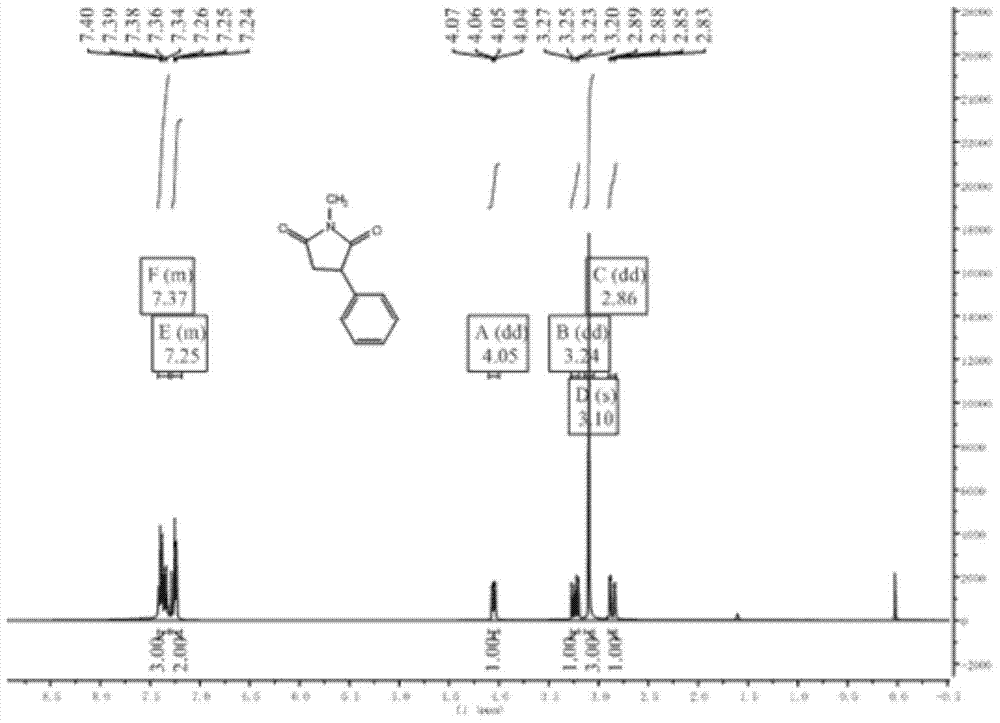
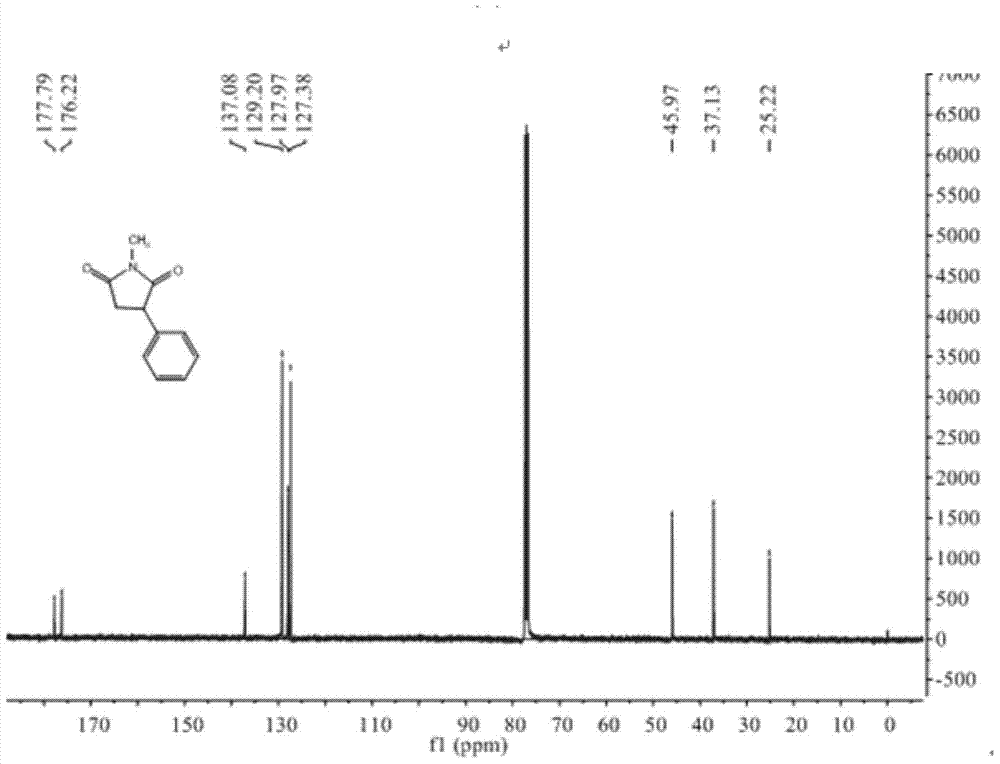
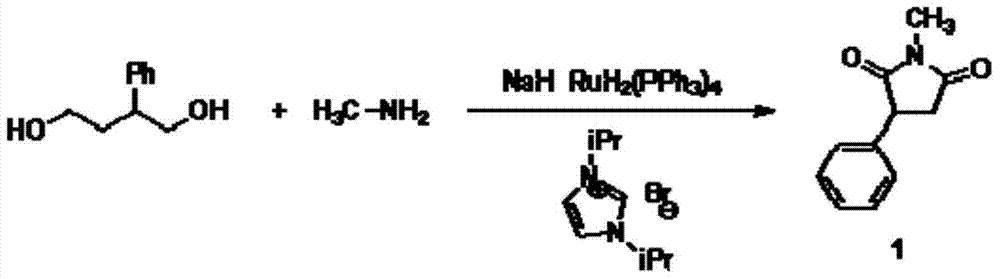
N-methyl-3 phenyl succinimide compound and preparation method thereof
The invention relates to an N-methyl-3 phenyl succinimide compound and a preparation method thereof. The structural formula of the compound is: the formula is described in the description. The preparation method comprises the following steps: with aniline as a raw material, reacting aniline with fluoboric acid and sodium nitrite at 5 DEG C to prepare a phenyl fluoroborate diazonium salt; reacting the phenyl fluoroborate diazonium salt with N-methylmaleimide in the presence of titanium trichloride with acetone / water as a solvent at minus 15 to 25 DEG C to obtain the N-methyl-3 phenyl succinimide compound. The yield of the N-methyl-3 phenyl succinimide compound prepared in the invention is high, the raw material is cheap and is easy to obtain, the reaction operation is simple, the reaction route is short, three wastes are few, and industrial production is easy to achieve.
Owner:DONGHUA UNIV
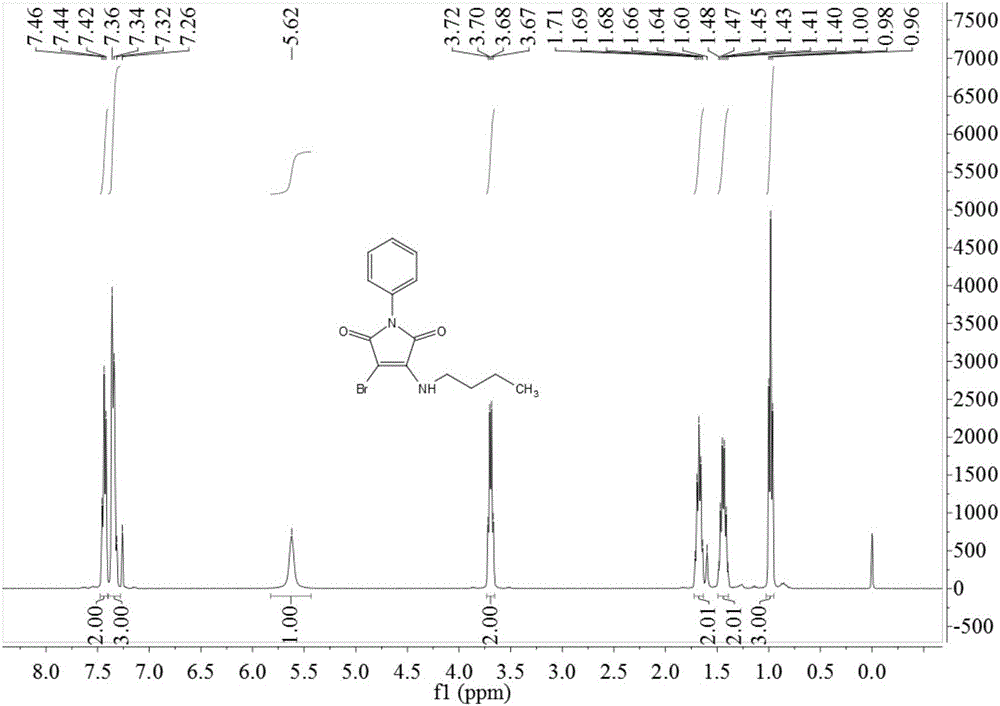
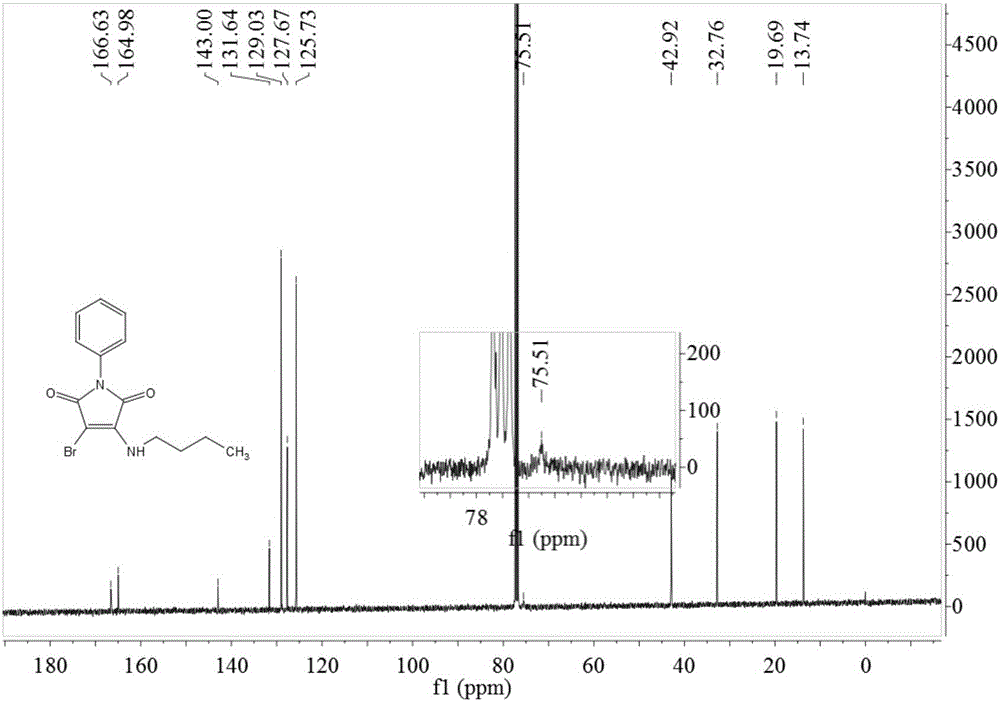
Preparation method of 3-n-butylamine-4-bromo-N-phenylmaleimide
InactiveCN106243009ADelayed reaction timeReduced responseOrganic chemistryN-phenylmaleimideWastewater
The invention relates to a preparation method of 3-n-butylamine-4-bromo-N-phenylmaleimide. the preparation method comprises the following steps: adding N-phenylmaleimide, n-butylamine and a brominating agent into a solvent, reacting at room temperature of minus 100 DEG C for 1-3 h, adding water and stirring after the end of the reaction, extracting, drying, concentrating, and recrystallizing to obtain 3-n-butylamine-4-bromo-N-phenylmaleimide. Yield of 3-n-butylamine-4-bromo-N-phenylmaleimide is high; reaction operation is simple; reaction route is short; and there is less ''three wastes'' (waste gas, wastewater and residue). The preparation method is easy for industrial production.
Owner:DONGHUA UNIV
Preparation method of 3,3,3-trifluoropropylene carbonate
The invention discloses a preparation method of 3,3,3-trifluoropropylene carbonate. The preparation method comprises the steps: with trifluoropropylene as a raw material, carrying out several simple reaction steps to obtain trifluoroepoxy propane, introducing carbon dioxide into a high-pressure reaction kettle by taking a metal conjugated microporous polymer complex as a catalyst, and preparing 3,3,3-trifluoropropylene carbonate at the temperature of 25-100 DEG C and the pressure of 0.1Mpa-3Mpa. Compared with the prior art, the method has the advantages that trifluoropropylene is taken as theraw material, so that the raw material cost is substantially lowered; and meanwhile, the yield is relatively high, and the catalyst can be repeatedly used, so that the method is suitable for industrial production.
Owner:陕西延长石油集团氟硅化工有限公司 +1
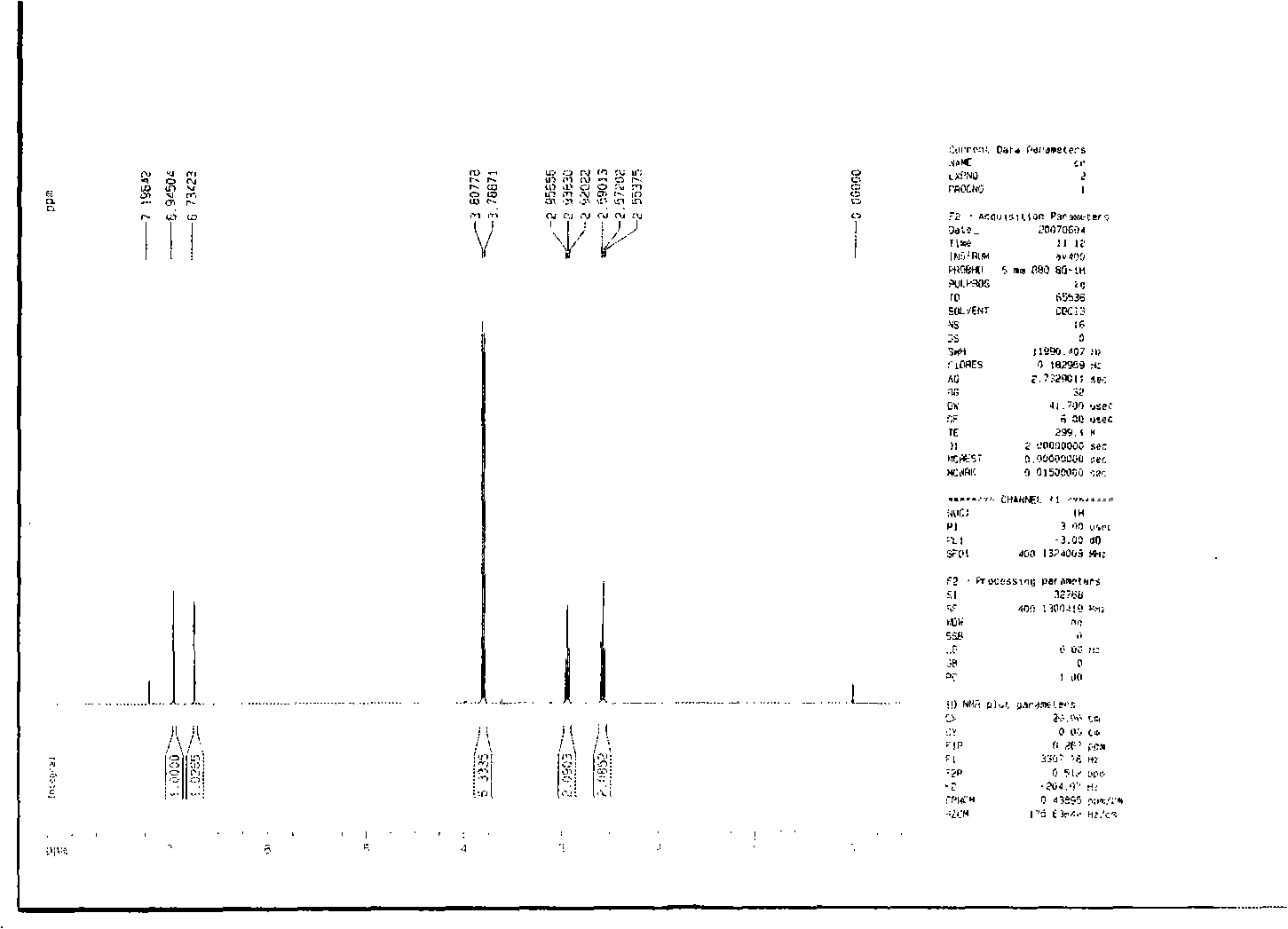
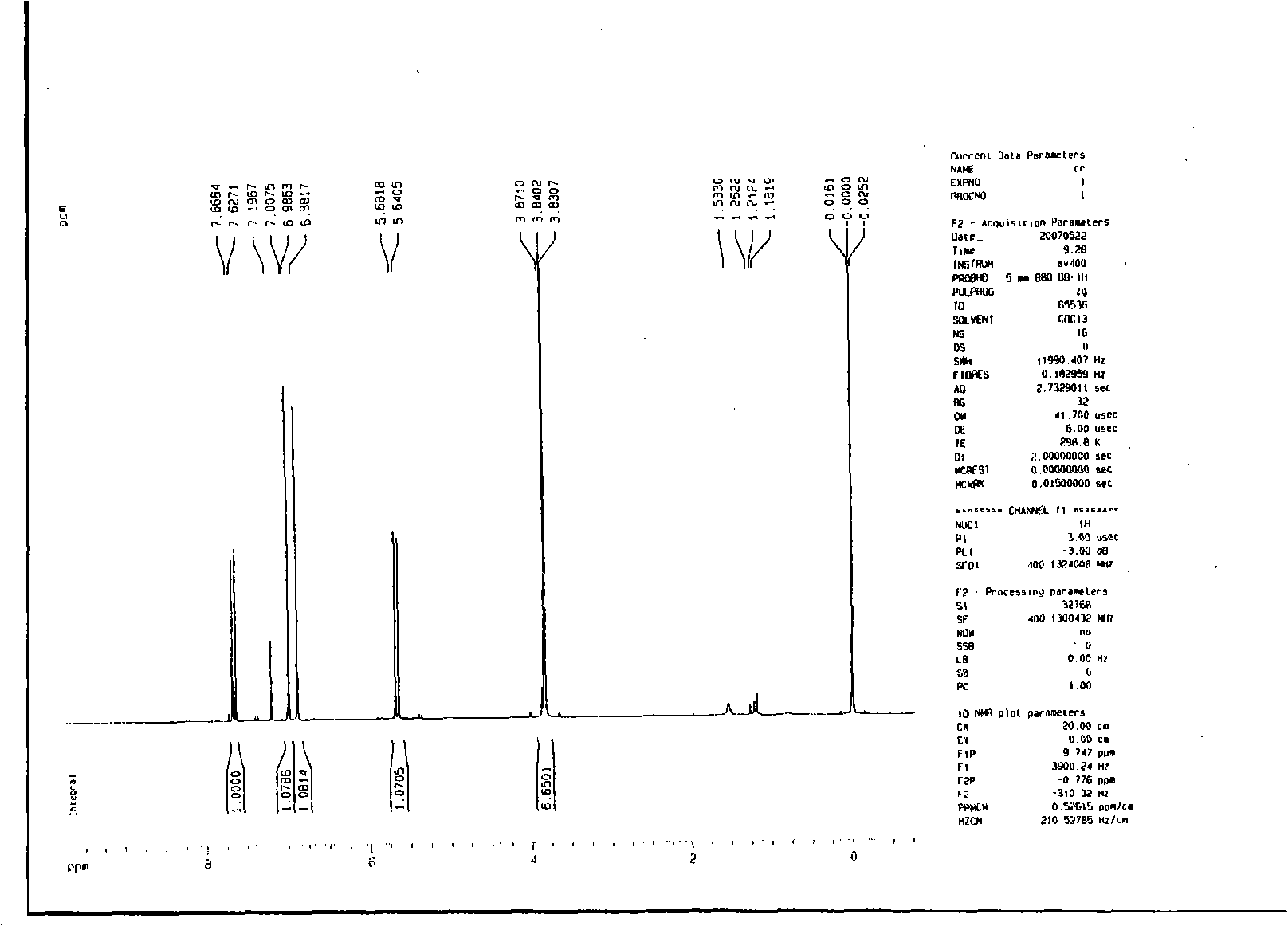
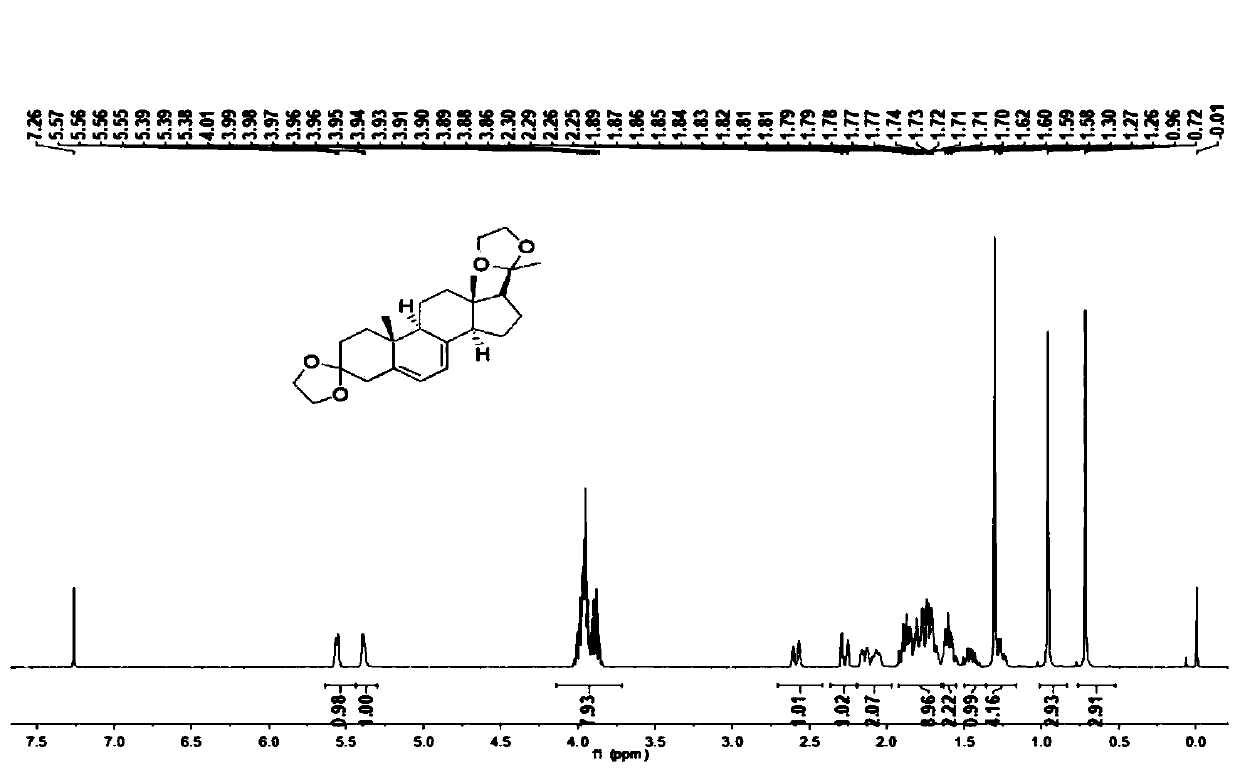
Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound
InactiveCN106117247AShort reaction timeShort reaction pathOrganic chemistryN-MethylmaleimideStructural formula
The invention relates to a preparation method of a 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound which has a following structural formula (please see the formula in the description). The preparation method comprises the steps that o-aminobenzenethiol, N-methylmaleimide and a catalyst are added into a solvent, oxygen is introduced, the materials are heated to 25 DEG C to 160 DEG C to react for 4 hours to 24 hours, purification is conducted, and then the 2-methyl-1,2,3,9-tetrahydro-benzo[b] pyrrole[1,4]-thiazine-1,3-diketone compound is obtained. According to the 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone prepared through the method, the yield is high, reaction operation is easy, the reaction route is short, an oxidizing agent is green and natural, few three wastes are generated, and industrialized production is easy to achieve.
Owner:DONGHUA UNIV
Preparation method and intermediate of deuterated calcitriol
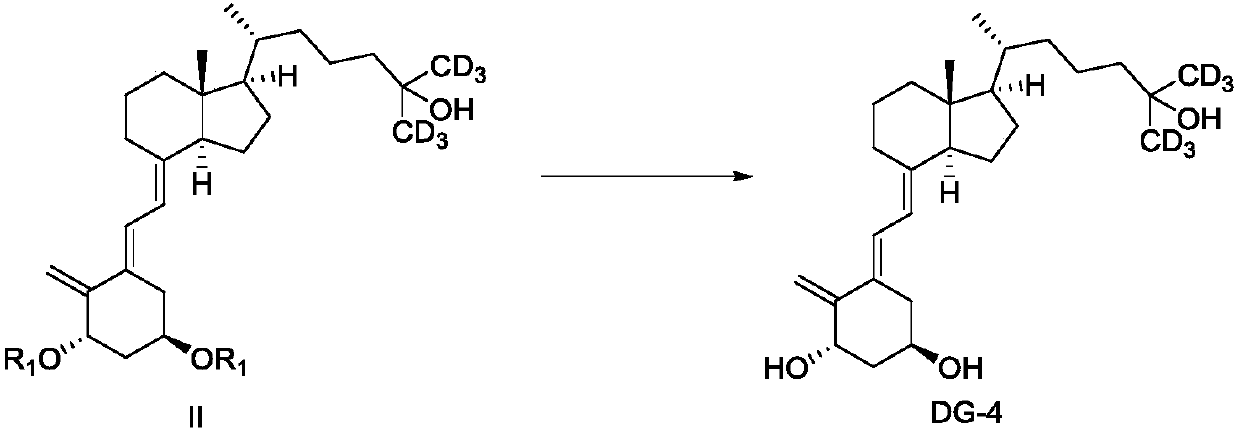
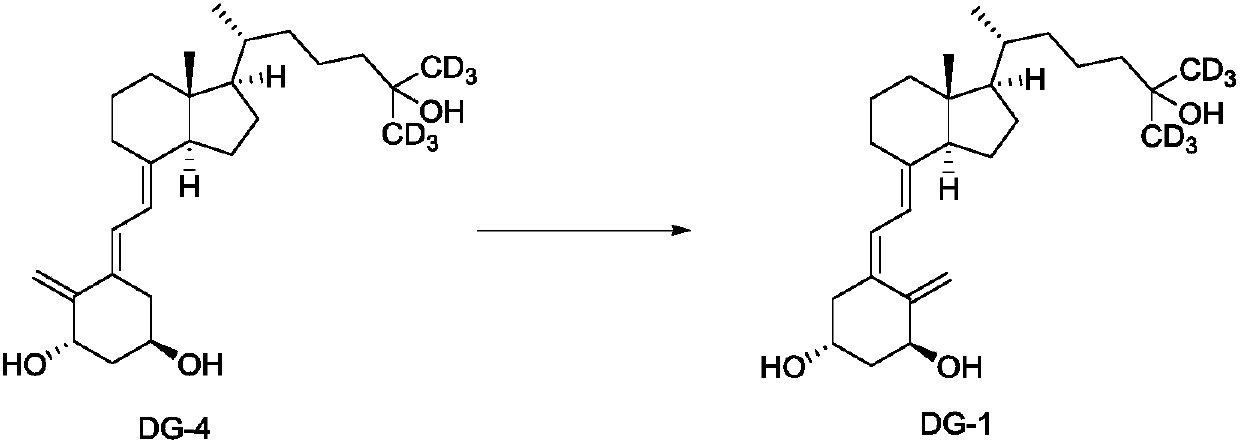
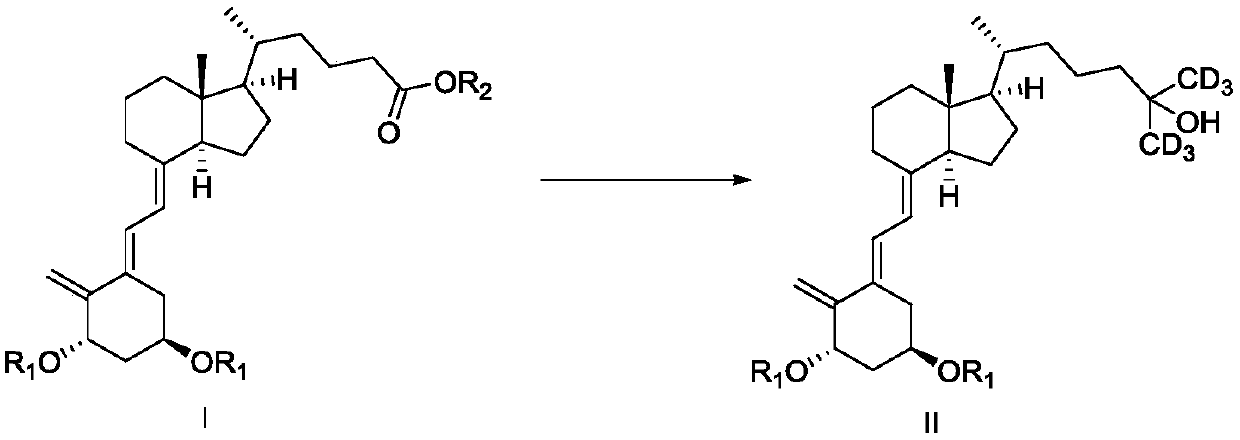
ActiveCN110885304AStarting materials are readily availableShort synthetic routeBulk chemical productionIsotope introduction to acyclic/carbocyclic compoundsPhotoisomerizationPhotosensitizer
The invention discloses a preparation method and an intermediate of deuterated calcitriol. The preparation method of deuterated calcitriol represented by formula DG-1 comprises the following steps: (1) carrying out a hydroxyl protecting group removal reaction shown in the description on a compound represented by formula II in an organic solvent 1 under the action of a deprotective agent to obtaina compound represented by formula DG-4; and (2) carrying out a photoisomerization reaction shown in the description on the compound of the formula DG-4 obtained in step (1) in an organic solvent 2 inthe presence of light irradiation and a photosensitizer to obtain the deuterated calcitriol of the formula DG-1, wherein R1 is a hydroxyl protecting group. The deuterated calcitriol preparation methodadopting the intermediate has the advantages of easily available initial raw materials, short synthesis route, simplicity in operation, and high yield.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Methylprednisolone preparation method
InactiveCN107840865AStarting materials are readily availableSimple lineSteroidsFermentationMethylprednisoloneDrug biotransformation
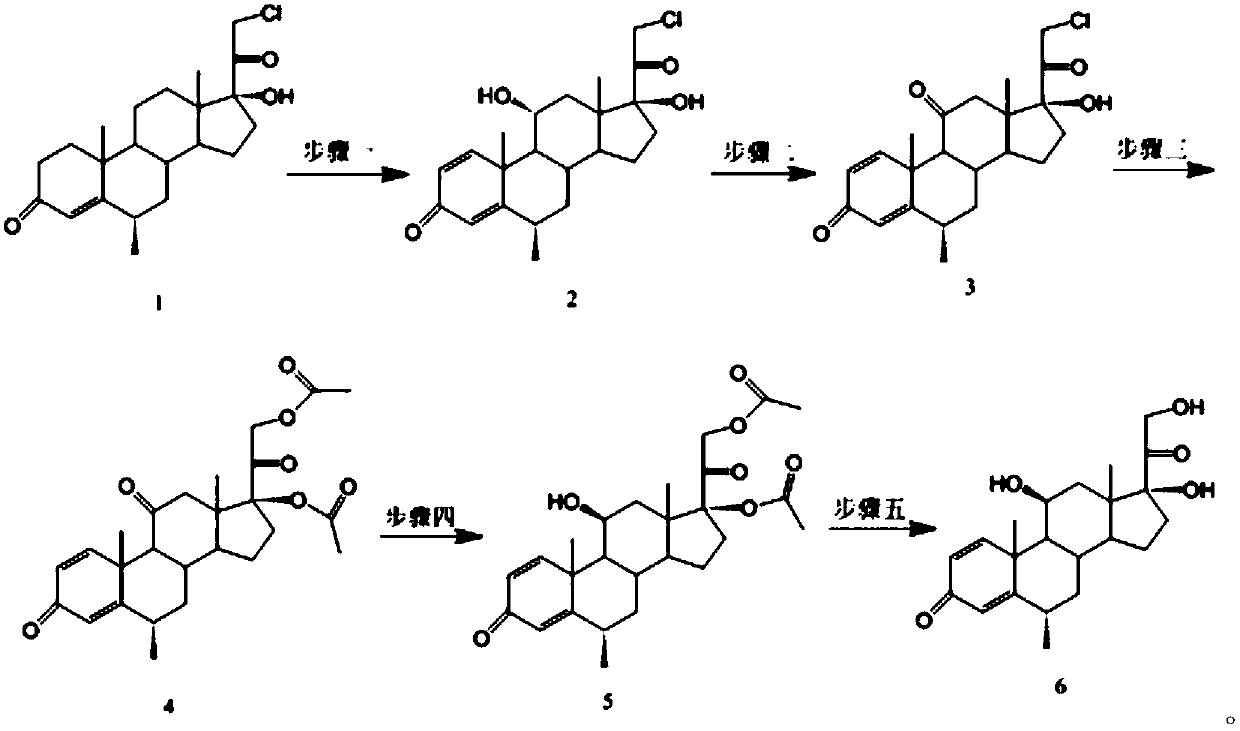
The invention discloses a methylprednisolone preparation method, wherein a compound represented by a formula 1 is used as a starting material, and biotransformation, oxidation, esterification, reduction and hydrolysis are sequentially performed to obtain the methylprednisolone.
Owner:TIANJIN JINYAO GRP
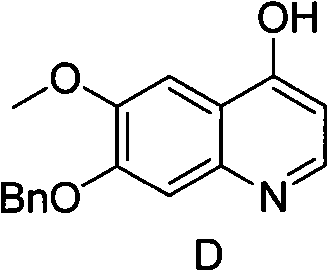
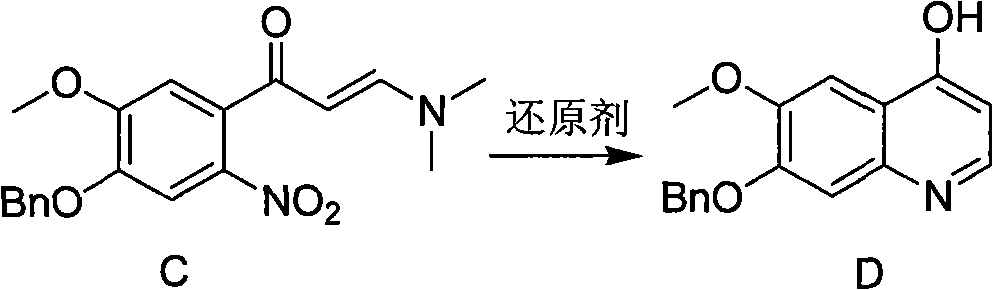
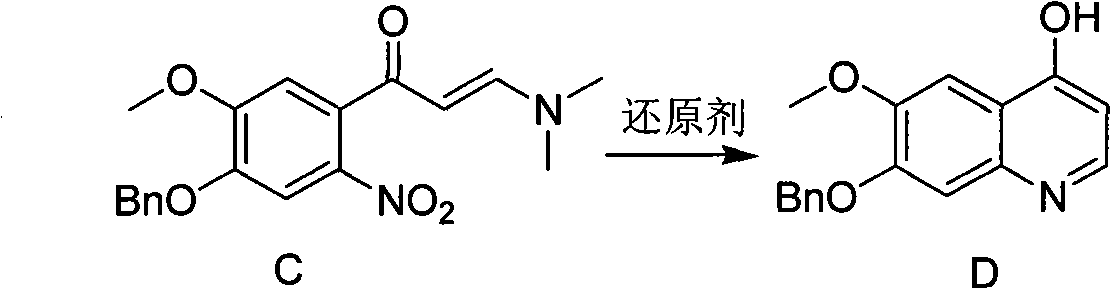
Synthesis method of 7- benzyloxy-6-methoxyl-4-hydroxyquinoline
The invention discloses a synthesis method of 7-benzyloxy-6-methoxyl-4-hydroxyquinoline, which is characterized by comprising the following step of: under the action of proton acid, performing nitro reduction reaction and cyclization reaction for a compound C and a reducing agent to obtain a compound D, namely 7-benzyloxy-6-methoxyl-4-hydroxyquinoline. The synthesis method has the advantages of simple operation, mild reaction condition, high safety, high reactions conversion rate and selectivity, low cost and good environmental-protection property and conforms to the green chemistry standard of atom economy. By adopting post-processing methods, such as filtering, crystallizing and the like, the synthesis method is simple, convenient and feasible, and is suitable for both small-scale preparation in a laboratory and large-scale industrialized production.
Owner:SHANGHAI CHEMPARTNER CO LTD
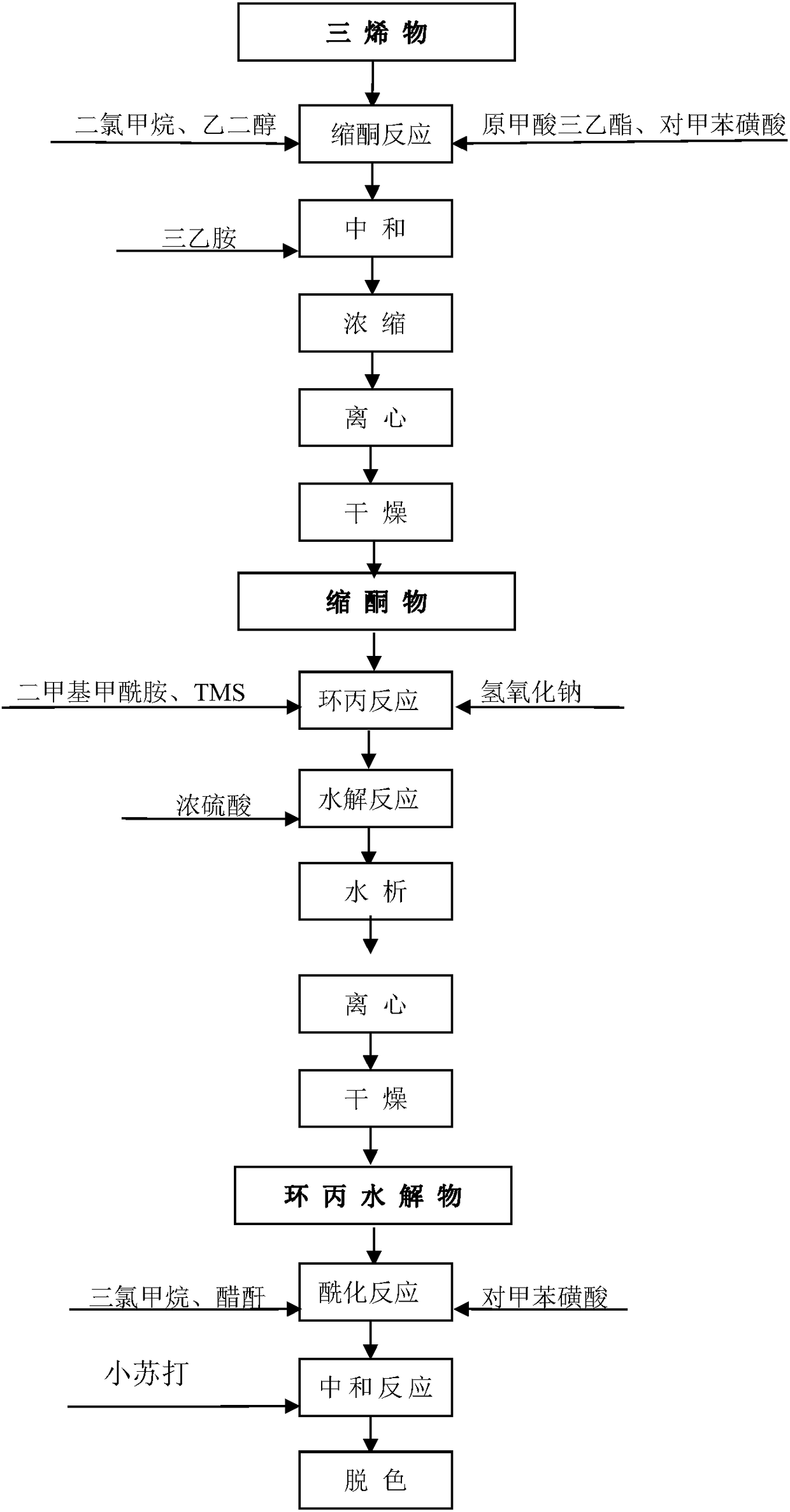
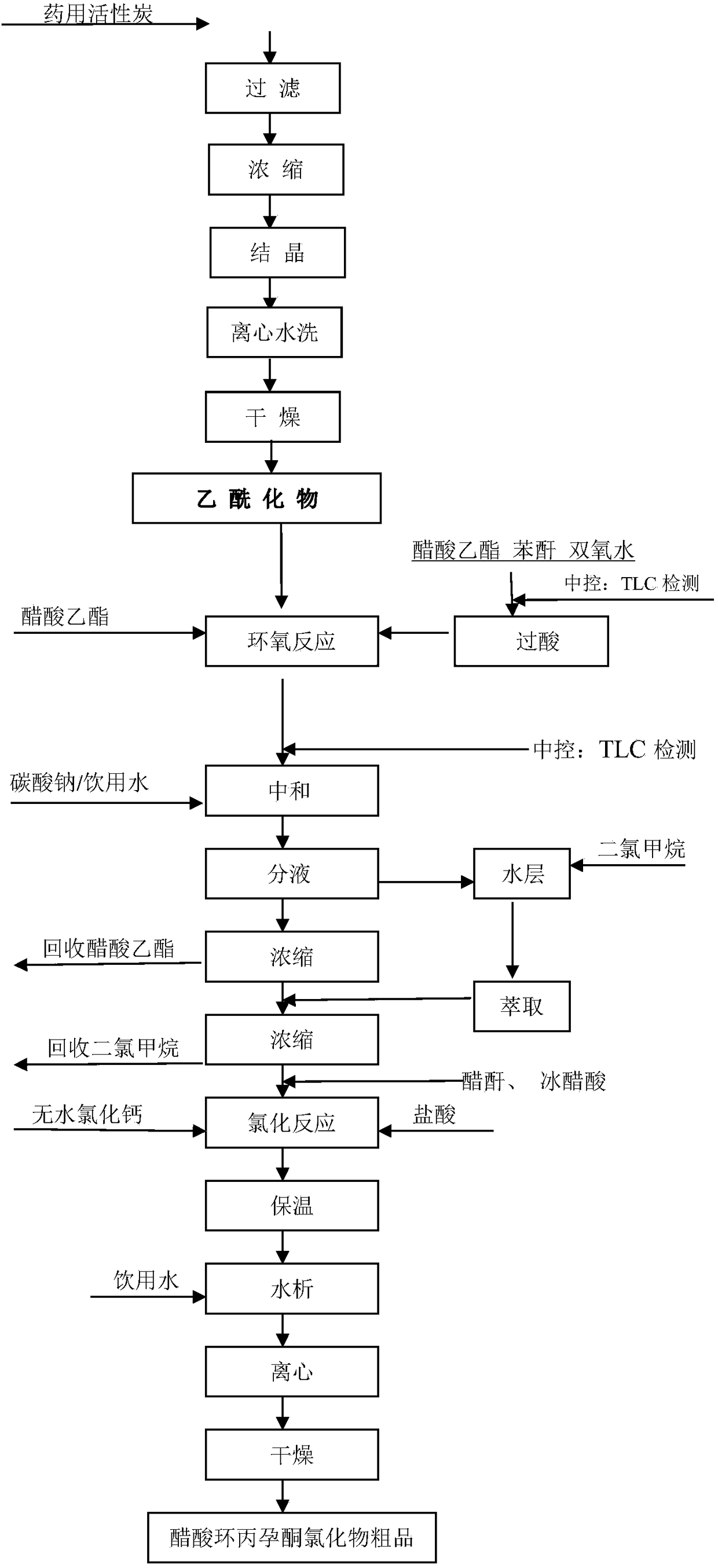
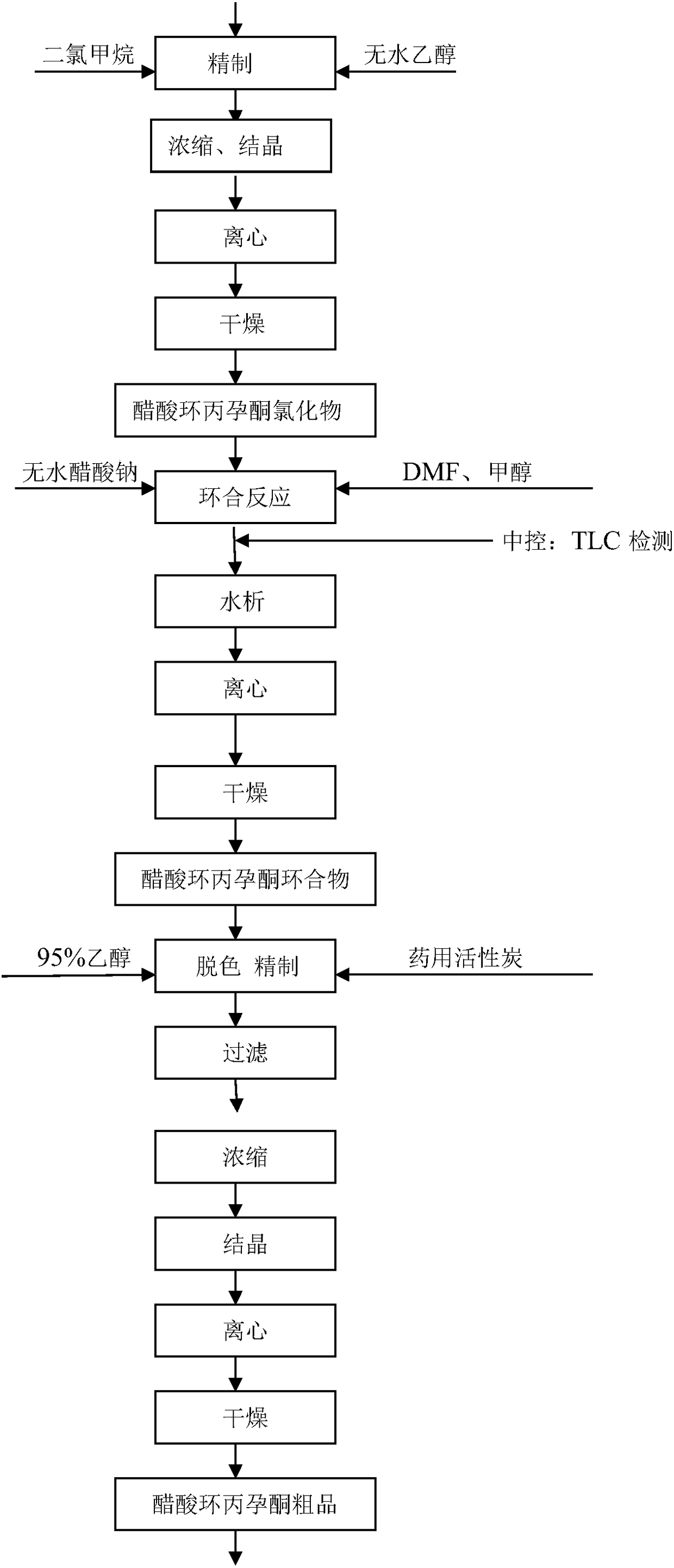
Preparation process of cyproterone acetate
The invention relates to a preparation process of cyproterone acetate. The preparation process of cyproterone acetate specifically comprises the following steps of ketalation reaction, cypro-hydrolysis reaction, acetyl reaction, epoxy reaction, chlorination reaction and cyclization reaction. The preparation process of cyproterone acetate can overcome the problem of benzene residues difficult to solve in original process products and is simple in initial raw materials, stable in reaction and high in total yield.
Owner:YUEYANG HUANYU PHARMA
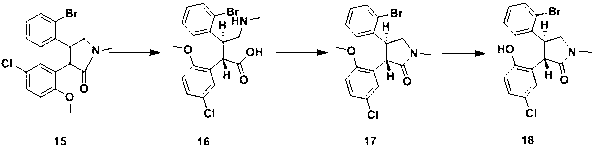
New process for synthesis of asenapine
ActiveCN102229613BMeet production requirementsStarting materials are readily availableOrganic chemistryMeth-Ptru catalyst
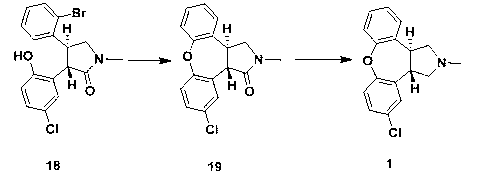
The invention discloses a process for synthesis of asenapine. The asenapine is prepared through adopting a compound (18) as a key intermediate and carrying out the following steps that: 1.1, the compound 18 is subjected to a Ullmann reaction under a alkaline condition through adopting copper powder as a catalyst to generate a ether (19); 1.2, the ether (19) is subjected to a carbonyl reduction to obtain the target compound of the asenapine (1). The process has the following advantages that: cheap and available 2-bromobenzaldehyde is adopted as an initial raw material and is subjected to acondensation, a addition, a reductive amination and a intramolecular cyclization reaction, a aminomethylation, a open loop transposition and then loop closing, a demethylation and a Ullmann loop closing reaction to synthesize of the asenapine (1); cis-trans-isomer is subjected to a delicate transposition to obtain a trans-product, such that the process is simplified and easy to be operated; the raw material is easy to be obtained and has cheap price; each reaction is a normal reaction, and reaction conditions are mild; a total yield is substantially improved; production cost is reduced; a purity of the product is more than 99% through a detection by HPLC.
Owner:安庆润科生物医药科技有限公司
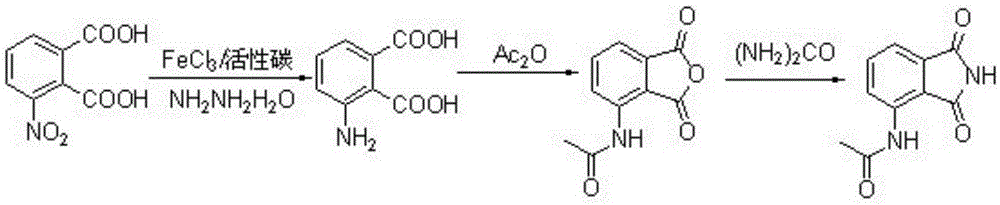
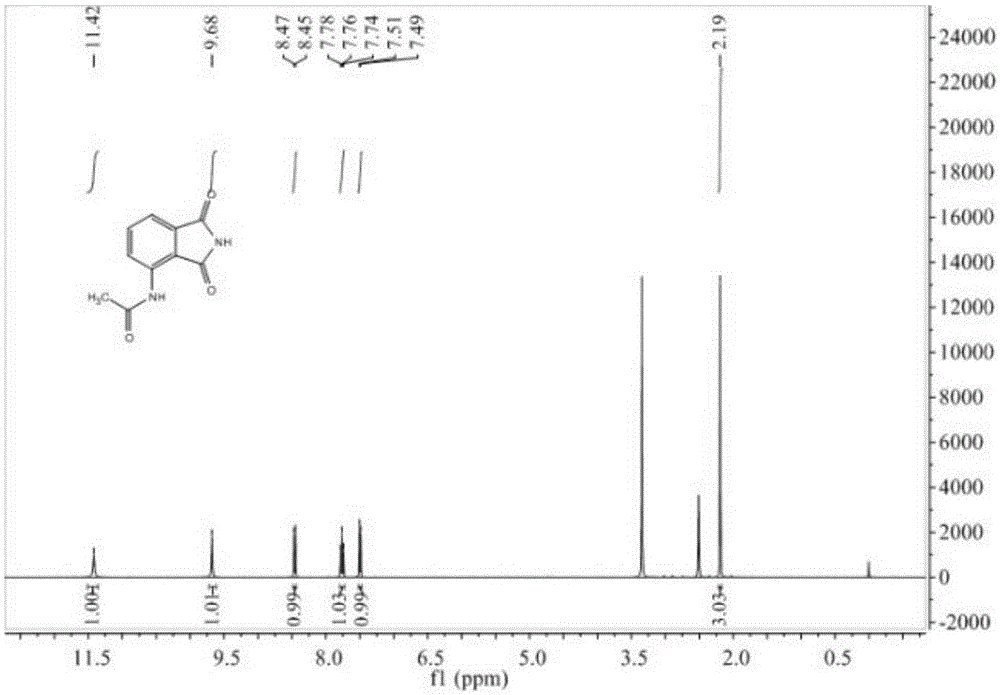
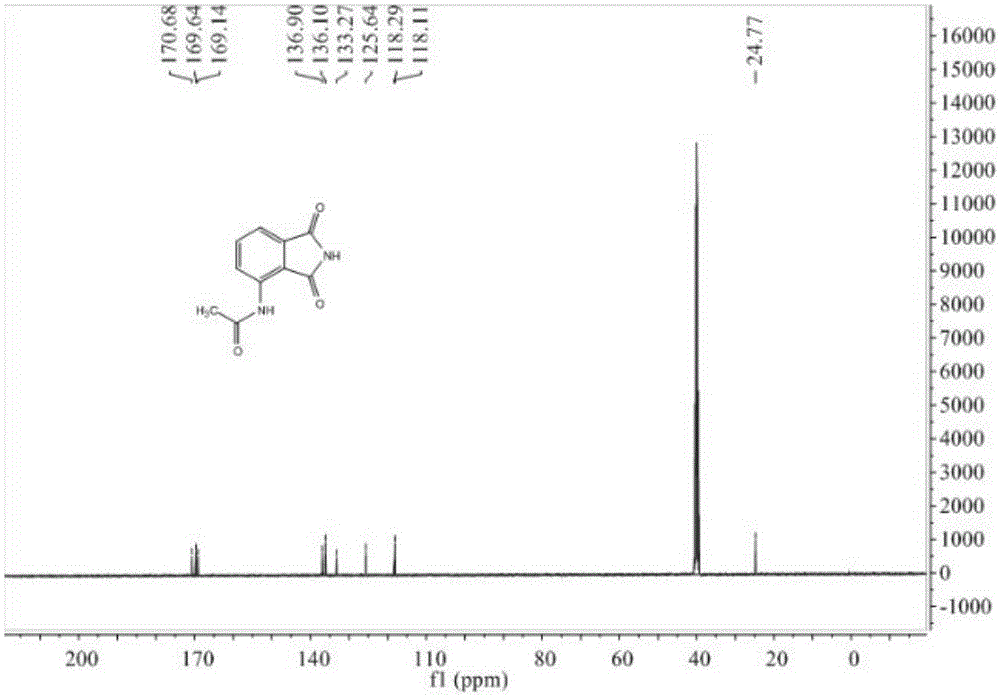
Preparation method of 3-acetyl aminophthalimide
InactiveCN105330587AShort reaction timeReduce waste disposalOrganic chemistryAcetic anhydrideAqueous sodium hydroxide
The invention relates to a preparation method of 3-acetyl aminophthalimide, comprising the following steps: dissolving 3-nitro phthalic acid in sodium hydroxide aqueous solution, then adding a reducer and a catalyst, heating to 70 to 80 DEG C, stirring and reacting for 1 to 20 hours, and recrystallizing to obtain 3-amino phthalic acid; stirring with acetic anhydride and performing reflux reaction for 1 to 10 hours to obtain N-(1,3-Dioxo-1,3-dihydro-2-benzofuran-4-yl)acetamide; dissolving the N-(1,3-Dioxo-1,3-dihydro-2-benzofuran-4-yl)acetamide in an organic solvent, adding an amino donor, and stirring and refluxing for 1 to 10 hours, thus obtaining the 3-acetyl aminophthalimide. The method is simple in operation, high in yield, short in reaction route, less in the three wastes (waste gas industrial residue), and easy for industrial production.
Owner:DONGHUA UNIV
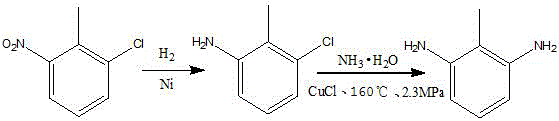
Preparation method of 2,6-diaminotoluene
ActiveCN106083599AShort chemical pathStarting materials are readily availableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSal ammoniacAnhydrous ethanol
The invention relates to the synthetic field of fine organic intermediates, specifically to a method for preparing 2,6-diaminotoluene by catalytic ammoniation. The method comprises the following steps: using anhydrous ethanol as a solvent, using 2,6-dichlorotoluene as a starting material, using ammonia water as an aminating agent, and heating and stirring the materials at normal pressure under the action of a palladium complex catalyst so as to prepare 2,6-diaminotoluene. By the synthetic method, high-temperature and high-pressure conditions in traditional technologies are avoided; economic cost is reduced, raw materials are simple and easy available, and reaction process is simple; and environmental protection is also considered, and raw materials harmful to the environment are avoided from being used in quantity.
Owner:山东川成医药有限公司
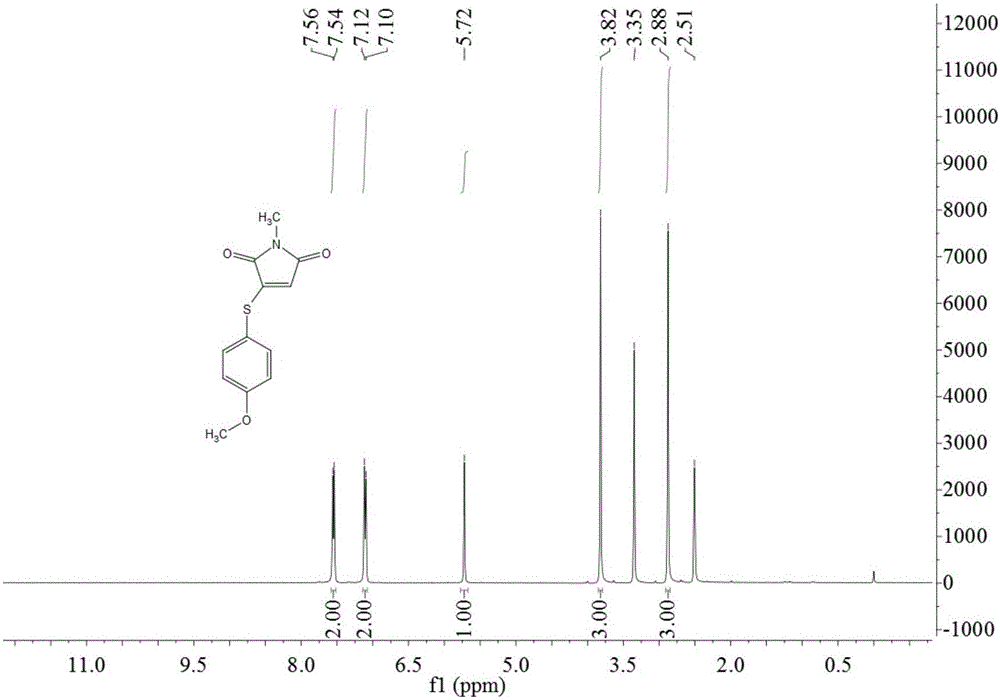
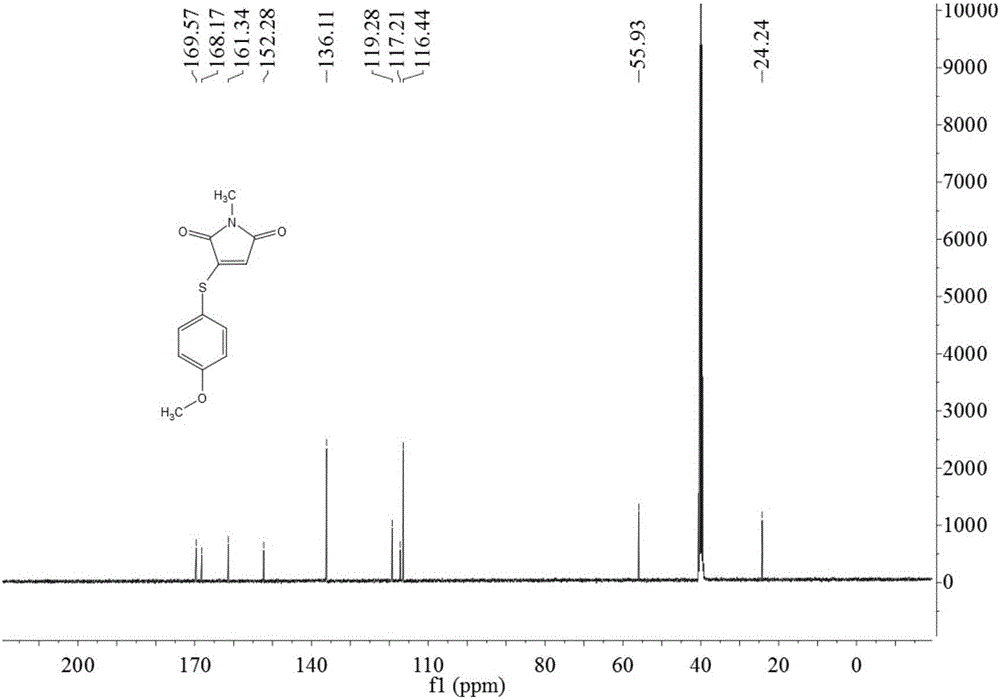
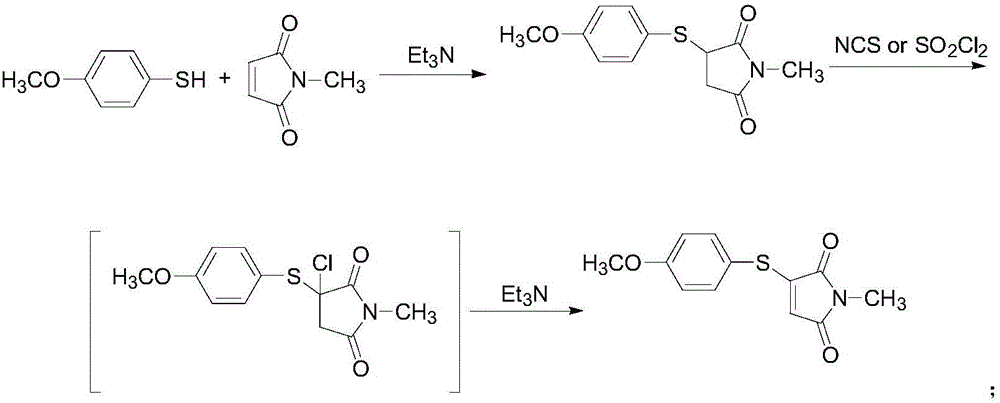
Preparation method of 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrolyl-2,5-dione compound
The invention relates to a preparation method of a 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrolyl-2,5-dione compound. The 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrolyl-2,5-dione compound has the structural formula shown in the description. The preparation method comprises the following steps: adding 4-methoxythiophenol, N-methylmaleimide, a copper salt catalyst and an additive into a reaction solvent, heating to 25-180 DEG C, enabling reaction for 4-24 hours and then purifying to obtain the 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrolyl-2,5-dione compound. The 3-(4-methoxyphenylthio)-1-methyl-1H-pyrrolyl-2,5-dione compound prepared by the preparation method provided by the invention is high in yield, simple in reaction operation, short in reaction route, less in generation of waste gas, waste liquid and waste solid and easy to achieve industrial production.
Owner:DONGHUA UNIV
Method for preparing 3-(4-methoxyphenyl)-succinimide
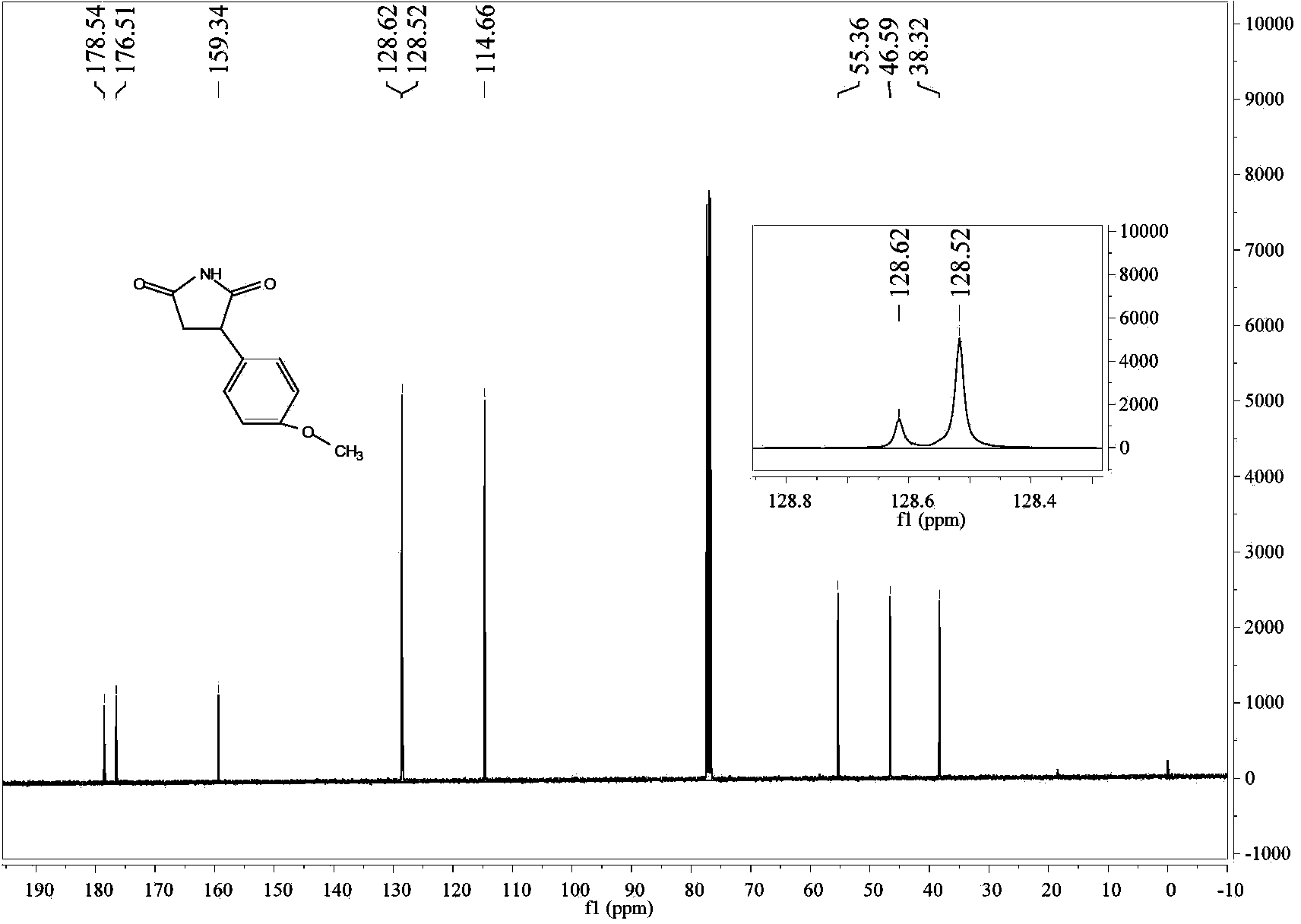
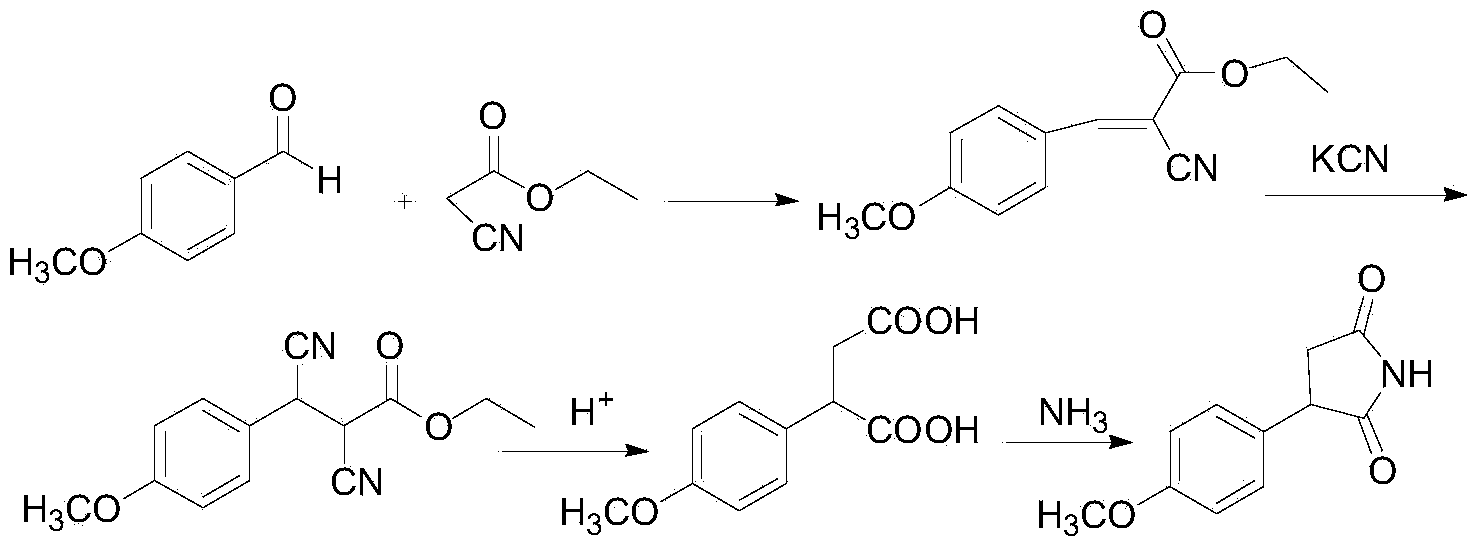
The invention relates to a method for preparing 3-(4-methoxyphenyl)-succinimide. The method comprises the following steps: dissolving anisole, maleimide and lewis acid into a solvent; heating and refluxing to react for 3-20 hours; adding 2N hydrochloric acid, stirring, filtering and recrystallizing to obtain 3-(4-methoxyphenyl)-succinimide. The 3-(4-methoxyphenyl)-succinimide prepared by the method has high yield, low price, simple reaction operation, short reaction route, less three-wastes, and easiness for realization of industrial production.
Owner:DONGHUA UNIV
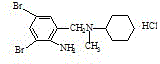
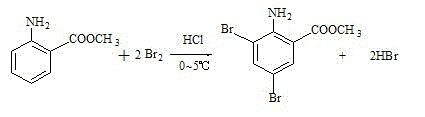
Synthesis production method of bromhexini hydrochloride
InactiveCN104610073AImprove performanceReduce pollutionOrganic compound preparationAmino compound preparationBenzoic acidPotassium borohydride
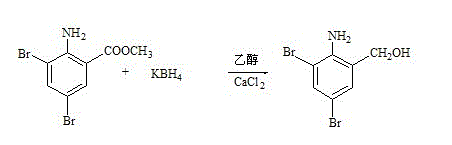
The present invention discloses synthetic production method of bromhexini hydrochloride. According to the method, methyl anthranilate is adopted as a starting raw material and is subjected to a bromine and hydrochloric acid bromination reaction to synthesize methyl 2-amino-3,5-dibromobenzoate, potassium borohydride and ethanol are adopted as raw materials to carry out a reduction reaction to synthesize 3,5-dibromo anthranilic alcohol, the 3,5-dibromo anthranilic alcohol and N-methyl cyclohexylamine are condensation to form bromhexine, hydrochloric acid is added to form a salt, a bromhexine hydrochloride crude product is synthesized, and re-crystallization with ethanol is performed to obtain the bromhexini hydrochloride. The method of the present invention has characteristics of short synthesis route, simple operation, easily available starting raw material, stable intermediate performance, high product yield and low production cost, and is suitable for industrial scale production.
Owner:KAMP PHARMA
Synthesis method of 2, 2-diaryl acetonitrile compound
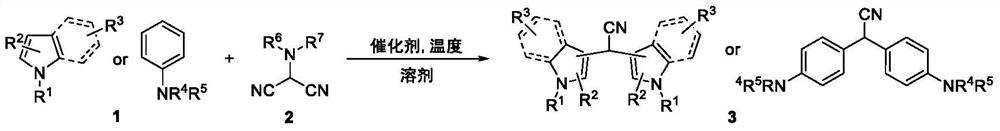
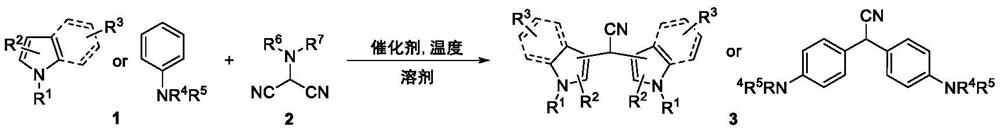
ActiveCN113444031ARapid responseStarting materials are readily availableCarboxylic acid nitrile preparationOrganic compound preparationCombinatorial chemistryAminomalononitrile
The invention relates to a synthesis method of a 2, 2-diarylacetonitrile compound, the synthesis method has the following reaction general formula: N, N-disubstituted aminomalononitrile and substituted aromatic hydrocarbon are subjected to a series coupling reaction under the action of a catalyst, and the 2, 2-diarylacetonitrile compound is rapidly and effectively synthesized. The method has the advantages of mild conditions, wide substrate range and convenience in operation, not only provides a new synthesis thought for synthesizing corresponding diindolylmethane (BIM) type functional molecules, but also greatly enriches a BIM compound library and promotes related activity research of the compounds.
Owner:LANZHOU UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
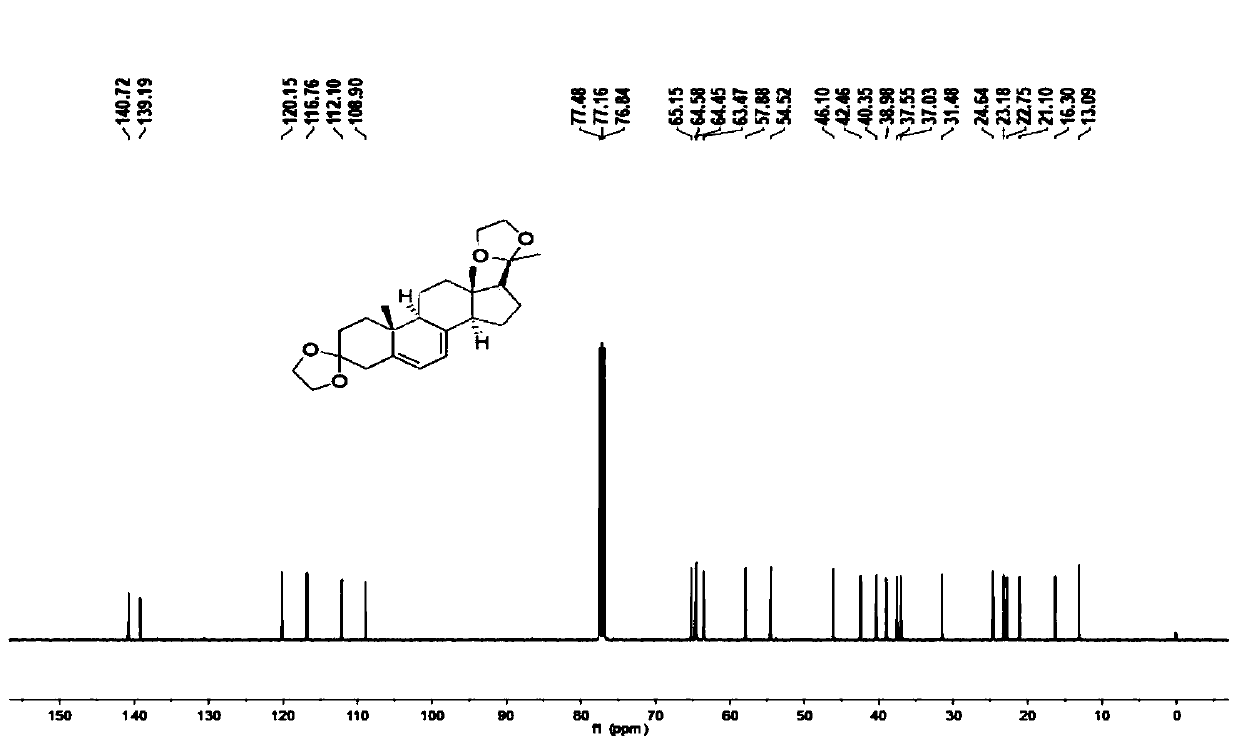
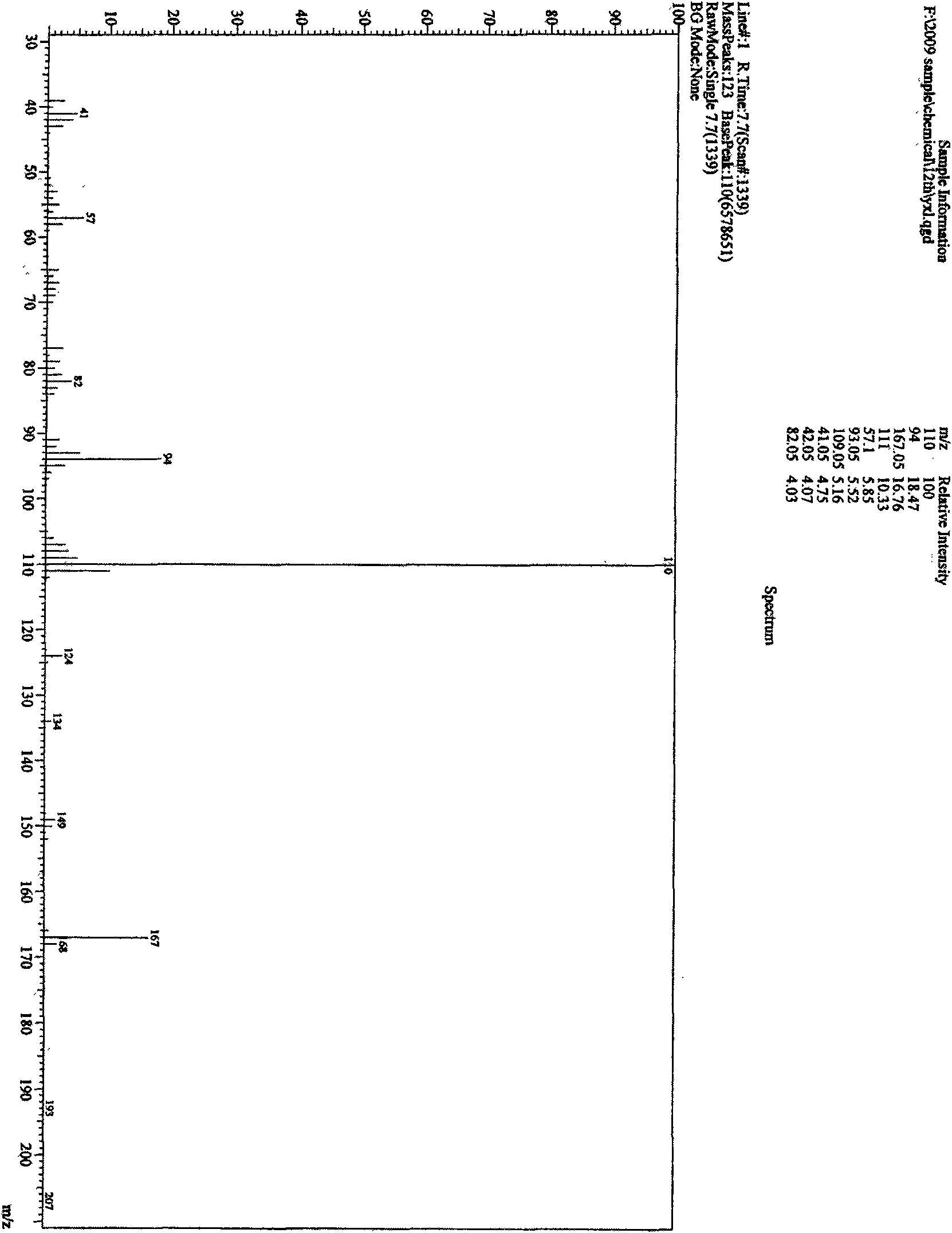
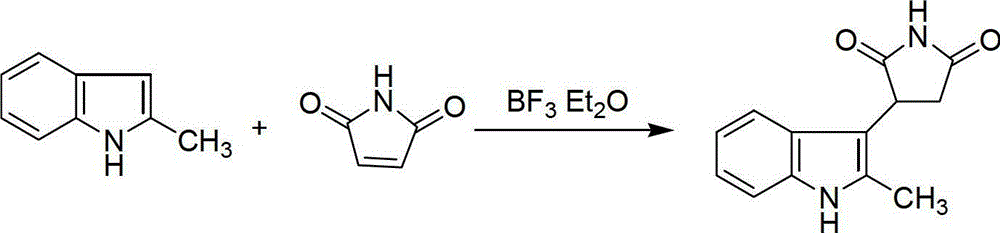
![Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound](https://images-eureka.patsnap.com/patent_img/96691672-4200-4107-8e84-e3e65e91e075/HDA0001035003560000011.PNG)
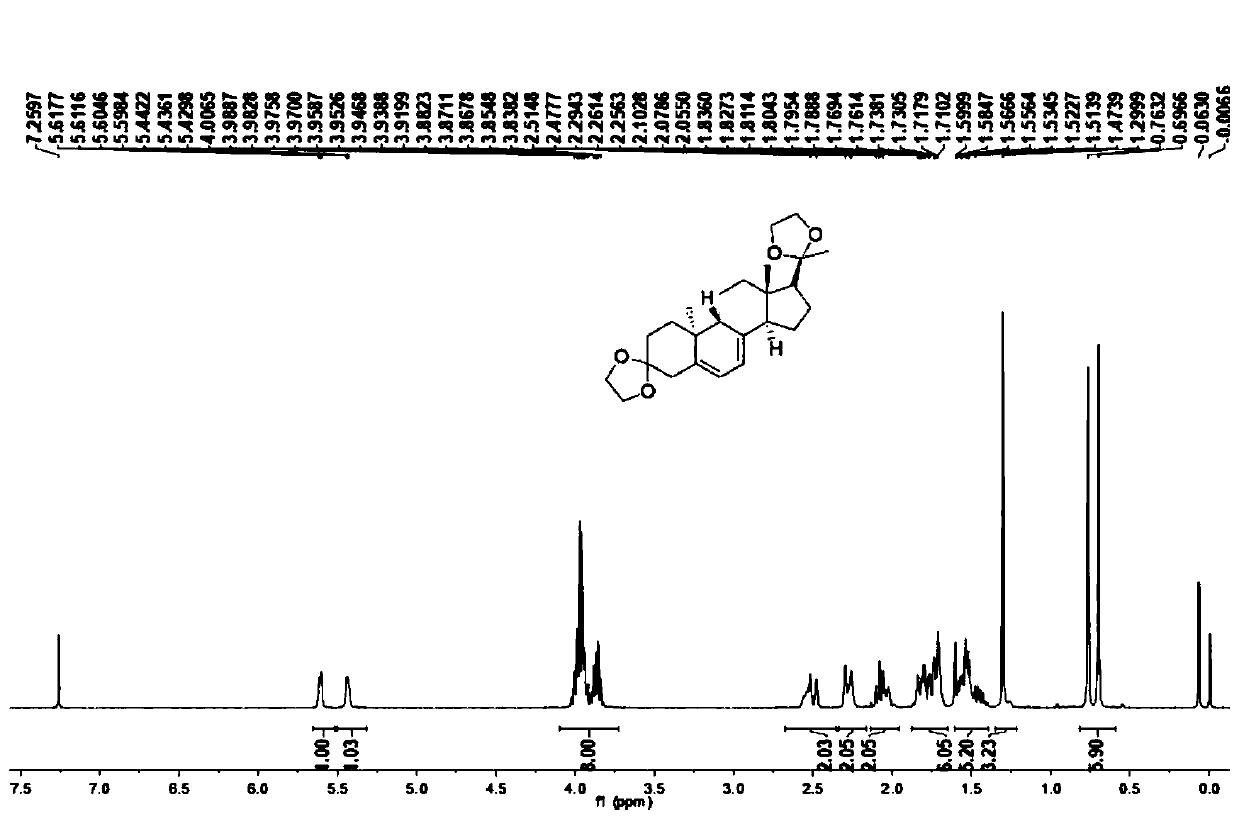
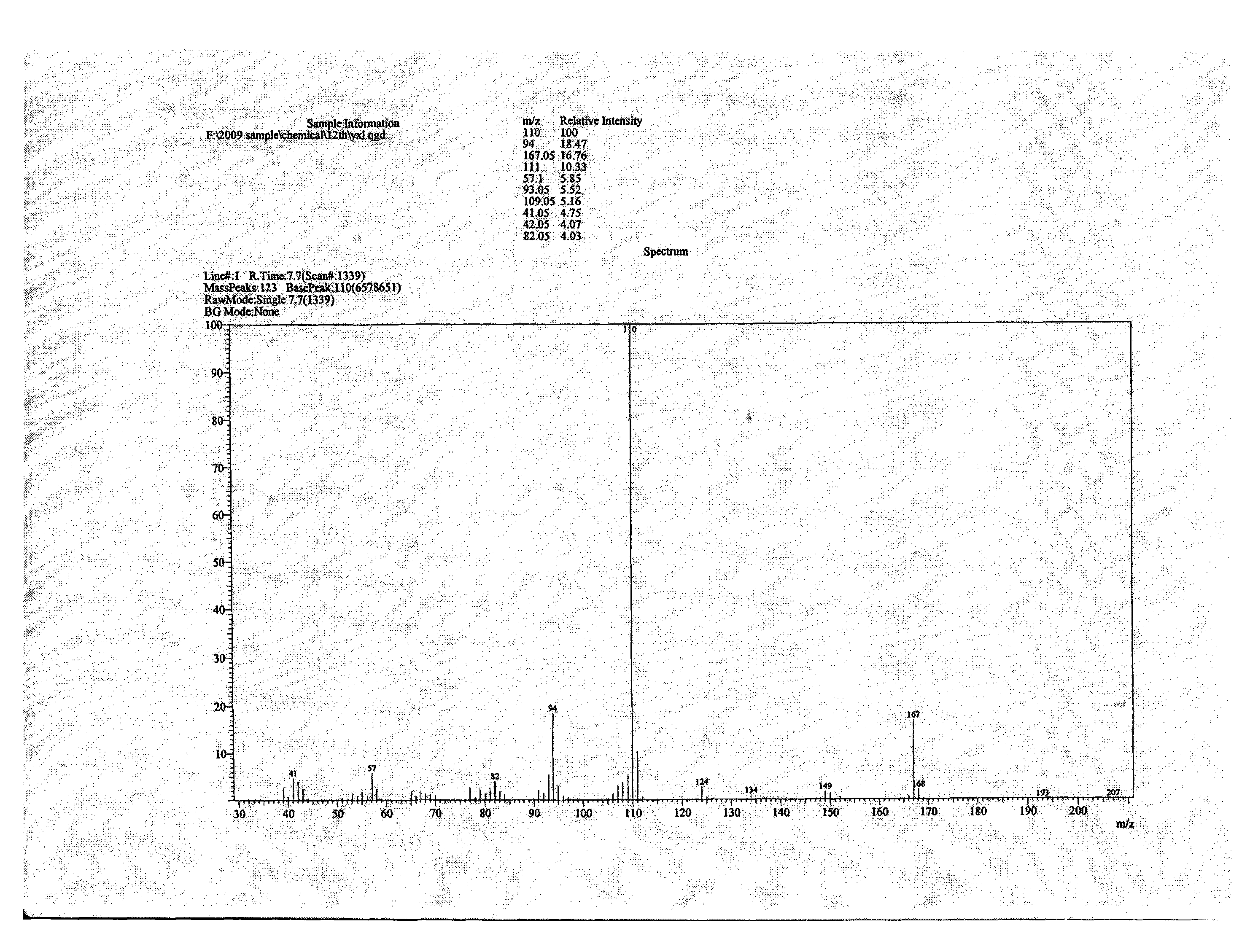
![Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound](https://images-eureka.patsnap.com/patent_img/96691672-4200-4107-8e84-e3e65e91e075/HDA0001035003560000012.PNG)
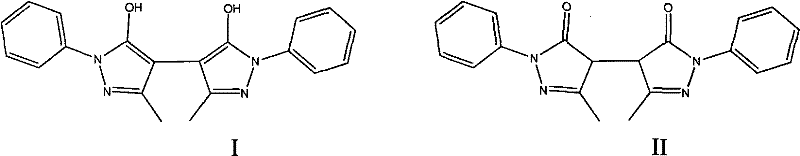
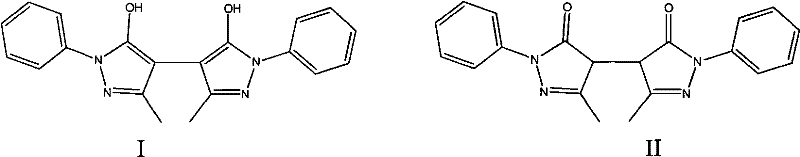
![Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound Preparation method of 2-methyl-1,2,3,9-tetrahydro-benzo[b]pyrrole[1,4]-thiazine-1,3-diketone compound](https://images-eureka.patsnap.com/patent_img/96691672-4200-4107-8e84-e3e65e91e075/BDA0001035003550000011.PNG)