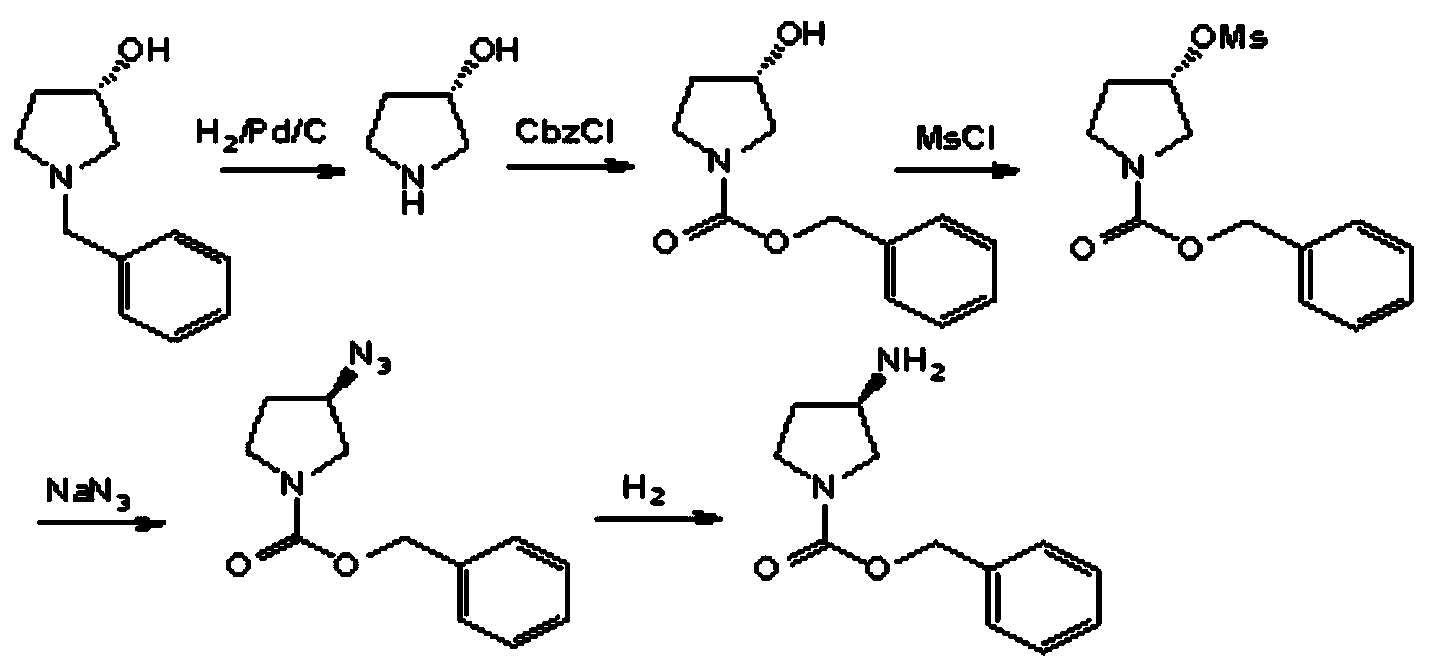
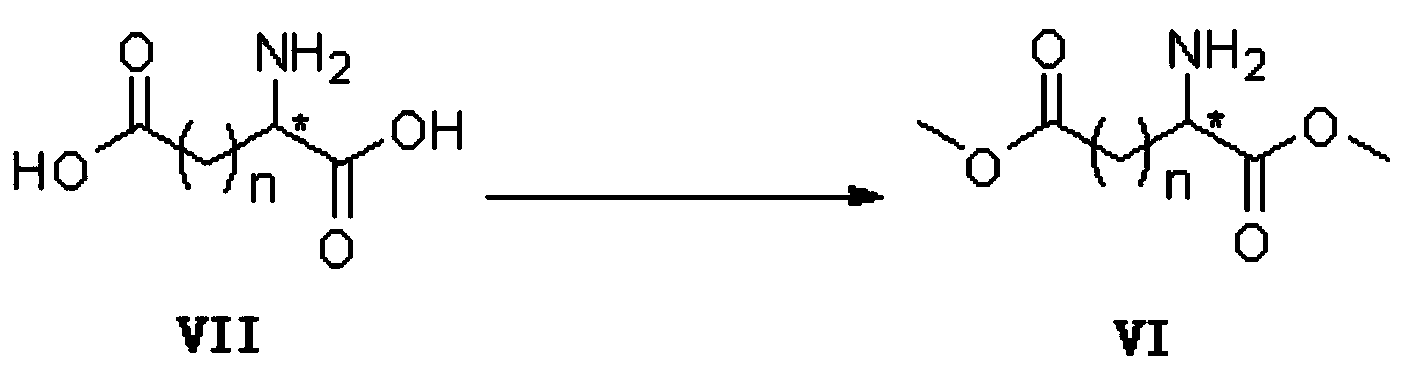
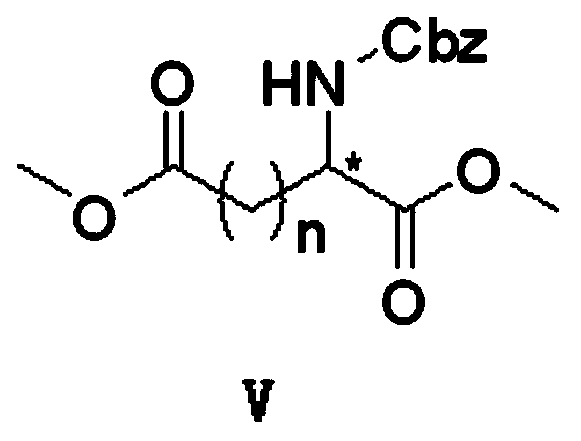
Preparation method of optical active medicine intermediate
A technology for compounds and hydrochlorides, applied in the field of preparation of optically active intermediates, can solve the problems of low resolution efficiency, low selectivity, and many by-products, etc., so as to reduce the risk of process changes, shorten the reaction conditions, and save costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] In a 250mL dry hydrogenation kettle equipped with a thermometer and mechanical stirring, add 12.4g of the compound of formula II (n=1), 0.65g (dry basis) of 10% palladium carbon, 156g of methanol and 24.3g of concentrated hydrochloric acid in sequence. Install the reaction kettle, first replace it with nitrogen three times, then replace it with hydrogen three times, pressurize to 1.0MPa, raise the temperature to 50-60°C and stir for 15-25 hours, stop the reaction after the raw materials are completely reacted, and cool to 20-30°C After filtration, the filtrate was distilled under reduced pressure at 60-65°C and 0.09MPa until there was no fraction, then 20g*3 isopropanol was added, and the isopropanol was distilled off under the same conditions to obtain a yellow solid. Heat to dissolve, then slowly drop 46.6g of ethyl acetate into methanol, after the addition, cool down to -10~-5°C to crystallize, filter, wash the filter cake with ethyl acetate, and dry to obtain the R-c...
Embodiment 2
[0050] Add 34.6g of benzylamine into a dried three-necked flask equipped with a thermometer, heat it to 45-55°C, add 35g of the compound of formula III (n=1) into the benzylamine, and keep the temperature at 45-55°C until the reaction of the raw materials is complete , to stop the reaction, quickly add 290g of water to the reaction bottle, stir for 2 hours, filter, transfer the filter cake into a 500ml reaction bottle, add 180g of water, stir for 2 hours, filter, dry the solid under reduced pressure until the water content is less than 1%, and obtain Compound of formula II (n=1) crude product 25.3g, yield 92%, purity: 94.2%, set aside.
[0051] Add 180g of petroleum ether and 25.3g of the crude product obtained above into a dried three-necked flask equipped with a thermometer, heat to 80-90°C and stir to dissolve, filter while hot, transfer the filtrate into another 500ml three-necked flask, and cool down to 20-90°C. Crystallize at 25°C, then cool to 0-5°C in an ice-water bath...
Embodiment 3
[0053]In a dry three-neck flask equipped with a dropping funnel and a thermometer, add 80g of compound of formula IV (n=1), 520g of dichloromethane and 102.3g of triethylamine, stir and cool down to -5~0°C, at this temperature Add dropwise the mixed solution of 97.6g methanesulfonyl chloride and 104g dichloromethane, after the raw materials have reacted, add 280g water, be warming up to 15~25 ℃ of layering, organic layer has saturated salt water washing, organic phase decompression distillation ( lower than 40°C, -0.09MPa) to obtain a yellow oil, cooled to 15-20°C, added 128g of ethanol, cooled to -10--5°C to crystallize, and obtained 106.4g of a white solid compound of formula III, with a yield of 83.1%, chemical purity 95.2%, optical purity 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com