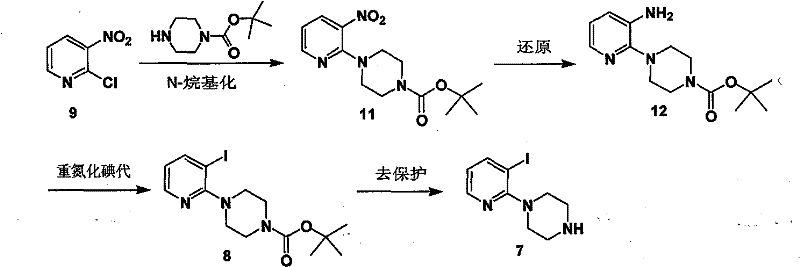
Method for synthesizing 4-(3-iodo-2-pyridinyl) piperazine compound
A synthetic method, pyridyl-based technology, applied in the direction of organic chemistry, can solve the problems of low overall yield, difficult product separation, by-product generation, etc., and achieve cheap starting materials, simple operation and post-treatment, and time-saving short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Synthesis of 4-(3-nitro-pyridine-2)-piperazine-1-tert-butyl carbonate
[0028] 2-Chloro-3-nitro-pyridine (47.5 g, 0.3 mol), N-tert-butoxy-piperazine (67 g, 0.36 mol), potassium carbonate (125 g, 0.9 mol) were added to dimethylsulfoxide ( 500 mL), stirred at room temperature for 10 minutes, then raised the temperature to 90° C., and reacted for 5 hours. After the system was cooled to room temperature, the reaction solution was poured into water and extracted with ethyl acetate (500 mL×3). The organic layers were combined, dried and concentrated to obtain tert-butyl 4-(3-nitro-pyridine-2)-piperazine-1-carbonate (87.5 g, yield 94.6%), which was used in the next reaction without purification.
[0029] 1 H NMR (300MHz, CDCl 3 ): δ1.41(s, 9H), 3.36-3.41(m, 4H), 3.48-3.51(m, 4H), 6.70-6.74(q, J=8.1Hz, J=4.5Hz, 1H), 8.06- 8.09(dd, J=8.1Hz, J=1.8,1H), 8.26-8.28(dd, J=4.5Hz, J=1.8,1H)
Embodiment 2
[0031]
[0032] Synthesis of 4-(3-nitro-pyridine-2)-piperazine-1-tert-butyl carbonate
[0033]2-Chloro-3-nitro-pyridine (4.75 g, 003 mol), N-tert-butoxy-piperazine (6.7 g, 0.036 mol), potassium carbonate (12.5 g, 0.09 mol) were added to acetonitrile (20 mL) , stirred at room temperature for 10 minutes, and then heated to reflux for 18 hours. After the system was cooled to room temperature, it was filtered, the filtrate was washed with water, and extracted with ethyl acetate (20 mL×3). The organic layers were combined, dried and concentrated to obtain tert-butyl 4-(3-nitro-pyridine-2)-piperazine-1-carbonate (7.5 g, yield 81%), which was used in the next reaction without purification.
[0034] 1 H NMR (300MHz, CDCl 3 ): δ1.41(s, 9H), 3.36-3.41(m, 4H), 3.48-3.51(m, 4H), 6.70-6.74(q, J=8.1Hz, J=4.5Hz, 1H), 8.06- 8.09(dd, J=8.1Hz, J=1.8,1H), 8.26-8.28(dd, J=4.5Hz, J=1.8,1H)
Embodiment 3
[0036]
[0037] Synthesis of 4-(3-nitro-pyridine-2)-piperazine-1-tert-butyl carbonate
[0038] 2-Chloro-3-nitro-pyridine (4.75g, 0.03mol), N-tert-butoxy-piperazine (6.7g, 0.036mol), cesium carbonate (19.5g, 0.06mol) were added to N,N -Dimethylformamide (20 mL), stirred at 60°C for 8 hours. After the system was cooled to room temperature, the reaction solution was poured into water and extracted with ethyl acetate (20 mL×3). The organic layers were combined, dried and concentrated to obtain tert-butyl 4-(3-nitro-pyridine-2)-piperazine-1-carbonate (8.5 g, yield 92%), which was used in the next reaction without purification.
[0039] 1 H NMR (300MHz, CDCl 3 ): δ1.41(s, 9H), 3.36-3.41(m, 4H), 3.48-3.51(m, 4H), 6.70-6.74(q, J=8.1Hz, J=4.5Hz, 1H), 8.06- 8.09(dd, J=8.1Hz, J=1.8,1H), 8.26-8.28(dd, J=4.5Hz, J=1.8,1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com