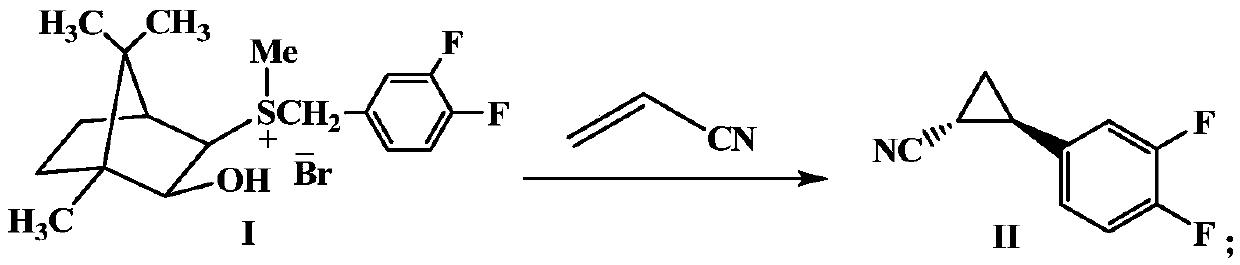
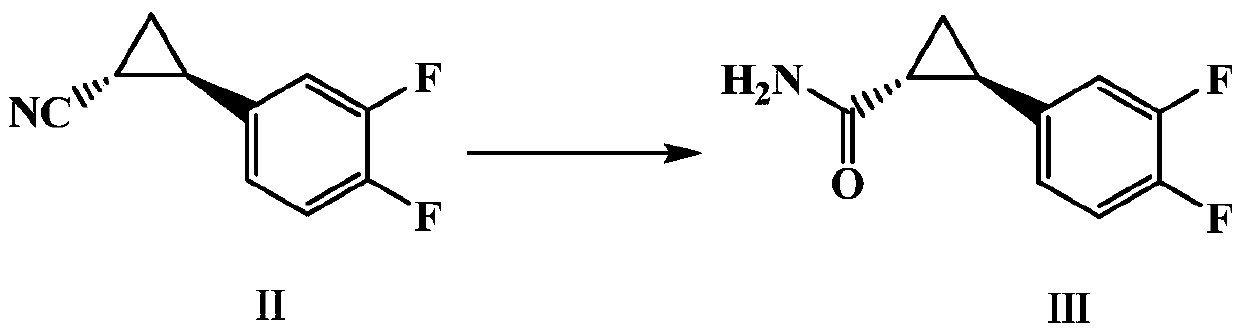
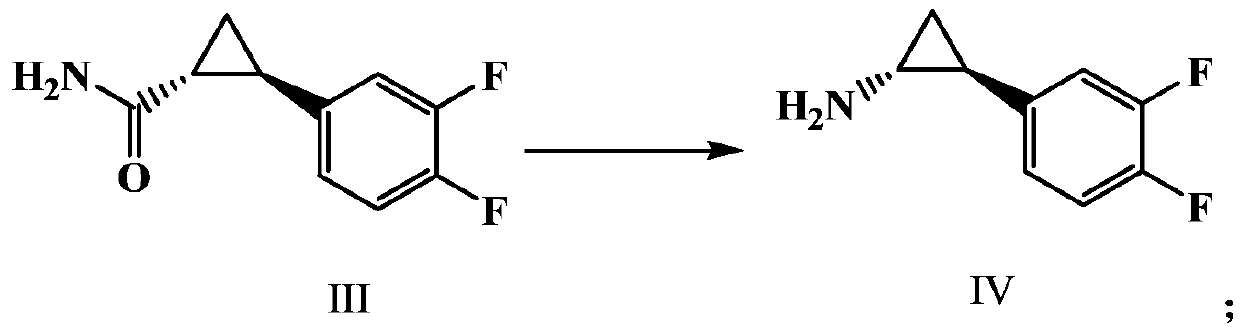
Preparation method of important intermediate of ticagrelor (1r, 2s)-2-(3,4-difluorophenyl)cyclopropylamine
A technology of difluorophenyl and ticagrelor, which is applied in the fields of organic synthesis and chemical medicine preparation, can solve the problems of poor reaction stereoselectivity, accidental damage, environmental pollution, explosive and harmful reagents, etc., and achieves excellent economic efficiency, The effect of shortening the process route and reducing the risk of accidental injury
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0042] Add camphorsulfonium ylide I (24.4g, 0.06mol), THF (300mL) and acrylonitrile (2.65g, 0.05mol) successively into a 500mL three-neck flask, place at -10°C, add potassium tert-butoxide (16.8 g, 0.15mol), after the addition was completed, the reaction was continued at -10°C, and the reaction was monitored by TLC. After the reaction was completed for about 12 hours, the reaction mixture was first filtered, and the filtrate was concentrated under reduced pressure to remove the solvent. Color oily product II 7.6g. The yield was 85.0%.
Embodiment 1-2
[0043] Embodiment 1-2. (1R,2R)-2-(3,4-difluorophenyl) the synthesis of cyclopropyl nitrile compound II
[0044] Add camphorsulfonium ylide I (24.4g, 0.06mol), THF (200mL) and acrylonitrile (2.65g, 0.05mol) successively into a 500mL three-neck flask, place at -78°C, add potassium tert-butoxide (16.8 g, 0.15mol), after the addition was completed, the reaction was continued at -78°C, and the reaction was monitored by TLC. After the reaction was completed for 24 hours, the reaction mixture was first filtered, and the filtrate was concentrated under reduced pressure to remove the solvent. The obtained crude product was colorless after simple removal of impurities. Oily product II 7.3 g. The yield was 82.0%.
Embodiment 1-3
[0045]Embodiment 1-3. (1R,2R)-2-(3,4-difluorophenyl) the synthesis of cyclopropyl nitrile compound II
[0046] Add camphorsulfonium ylide I (20.3g, 0.05mol), methyltetrahydrofuran (200mL) and acrylonitrile (2.65g, 0.05mol) in sequence in a 500mL three-neck flask, place at -50°C, and add potassium tert-butoxide (16.8g, 0.15mol), after the addition was completed, the reaction was continued at -50°C, and the reaction was monitored by TLC. After the reaction was completed for 20 hours, the reaction mixture was first filtered, and the filtrate was concentrated under reduced pressure to remove the solvent. The obtained crude product was obtained by simple removal of impurities. Colorless oily product II 8.5g. The yield was 84%.
[0047] Then use the (1R,2R)-2-(3,4-difluorophenyl)cyclopropylnitrile compound II prepared in any of the above Examples 1-1~1-3 for the synthesis of (1R,2R)- 2-(3,4-difluorophenyl) cyclopropyl formamide III, specific examples are as follows:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com