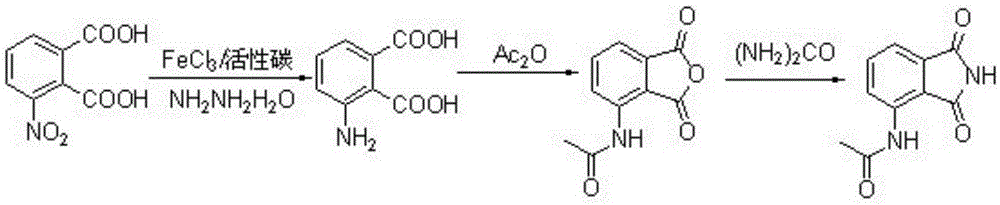
Preparation method of 3-acetyl aminophthalimide
A technology based on phthalimide and nitrophthalic acid, which is applied in the field of preparation of pharmaceutical and chemical intermediates, can solve the problems of long reaction route, high production cost and low yield, and achieve short reaction route, The effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 40.8g (0.19mol) of 3-nitrophthalic acid and 250mL of 3M sodium hydroxide solution into a 1000mL three-necked flask, heat and stir until the reaction liquid becomes clear at 70°C; add 3.2g (12mmol) of Crystal water, 4.0 g of ferric chloride and activated carbon, stirred for 15 min; finally, 26 mL (0.53 mol) of 85% hydrazine hydrate was added dropwise, and the addition was completed in 30 min. The reaction was tracked by TLC and reacted for 6 h to obtain 3-aminophthalic acid. After the reaction was completed, it was filtered with suction, and the filtrate was adjusted to pH=3 by adding 4N hydrochloric acid, and a large amount of solids were precipitated. Stir at room temperature for 10 min, filter with suction, wash the solid with water, and recrystallize from ethanol to obtain 28.1 g of a yellow-white solid with a yield of 80.1%. mp: 172-174°C. IR (KBr): 3430, 1715, 1634, 1527cm -1 ; 1 HNMR (400MHz, DMSO-d 6 ): 6.68(d, J=7.3Hz, 1H), 6.84(d, J=8.3Hz, 1H), 7.05~7.2...
Embodiment 2
[0036]Add 28.0 g (0.15 mol) of 3-aminophthalic acid obtained in Example 1 into a 250 mL one-necked flask, then add 100 mL of acetic anhydride, and heat to reflux. The reaction was tracked by TLC, and the reaction was carried out for 4 hours to obtain 3-acetamidophthalic anhydride. After the reaction was completed, the reaction bottle was placed in an ice-water bath and stirred for 15 minutes, and a solid precipitated out; added diethyl ether to stir, suction filtered and washed with diethyl ether to obtain a light yellow flaky solid, which was weighed after drying to obtain 24.9 g, with a yield of 78.5%. . mp: 180-182°C. IR (KBr): 3351, 1841; 1766; 1700; 1603cm -1 . 1 HNMR (400MHz, CDCl 3 ):2.34(s,3H),7.69(d,J=7.3Hz,1H),7.82~7.93(m,1H),8.97(d,J=8.5Hz,1H),9.12(s,1H).MS (EI):205[M + ].
Embodiment 3
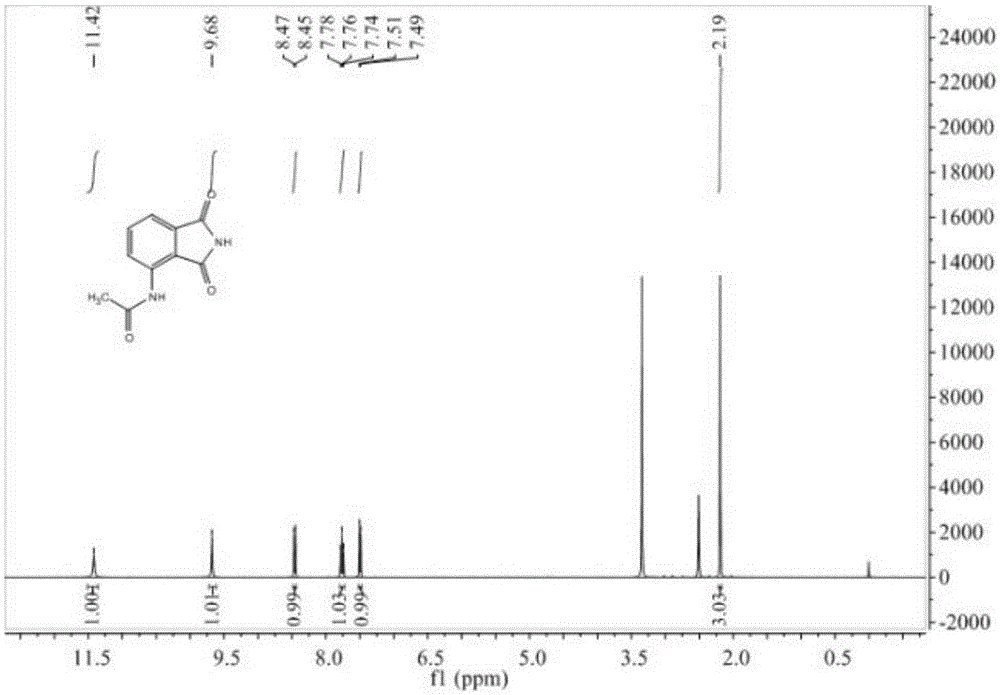
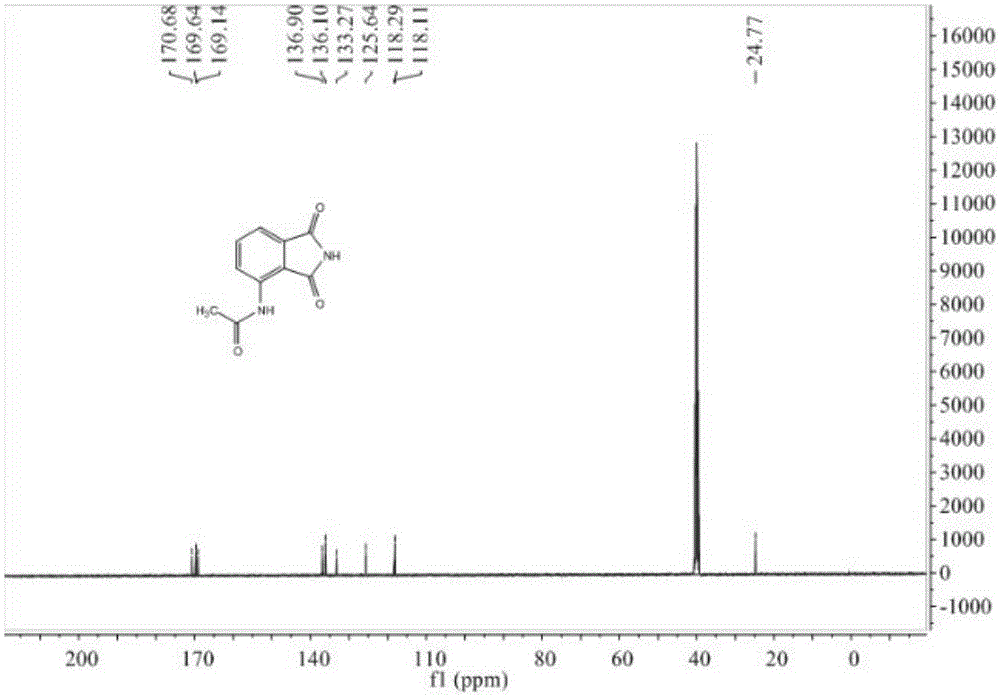
[0038] 24.9 g (0.12 mol) of 3-acetamidophthalic anhydride obtained in Example 2 and 15.0 g (0.25 mol) of urea were respectively added to a 250 mL round bottom flask, and DMF 100 mL was added. Heated to 100°C, followed by TLC for 6 hours to obtain 3-acetamidophthalimide. After the reaction was completed, cool to room temperature, add 100 mL of water, stir for 15 min, and a solid precipitated; suction filtered, washed the solid with water, and recrystallized with 95% ethanol to obtain 2.1 g of a yellow-white solid with a yield of 85%. mp: 238~240℃. IR (KBr): 3301, 1762, 1690, 1609, 1523, 1296cm -1 ; 1 HNMR (400MHz, DMSO-d 6 ):δ2.19(s,3H),7.50(d,J=7.3Hz,1H),7.76(t,J=7.8Hz,1H),8.46(d,J=8.4Hz,1H),9.68(s ,1H),11.42(s,1H); 13 CNMR (101MHz, DMSO-d 6 ): δ24.77, 118.11, 118.29, 125.64, 133.27, 136.10, 136.90, 169.14, 169.64, 170.68. MS(EI): 204[M + ],like figure 2 and image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com