Preparation process of cyproterone acetate
A technology for the preparation of cyproterone acetate, which is applied in the chemical industry, can solve the problems of not being well adapted to industrial production, benzene residues, and low total yields, and achieves overcoming the problems of benzene residues, stable reactions, and high total yields. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
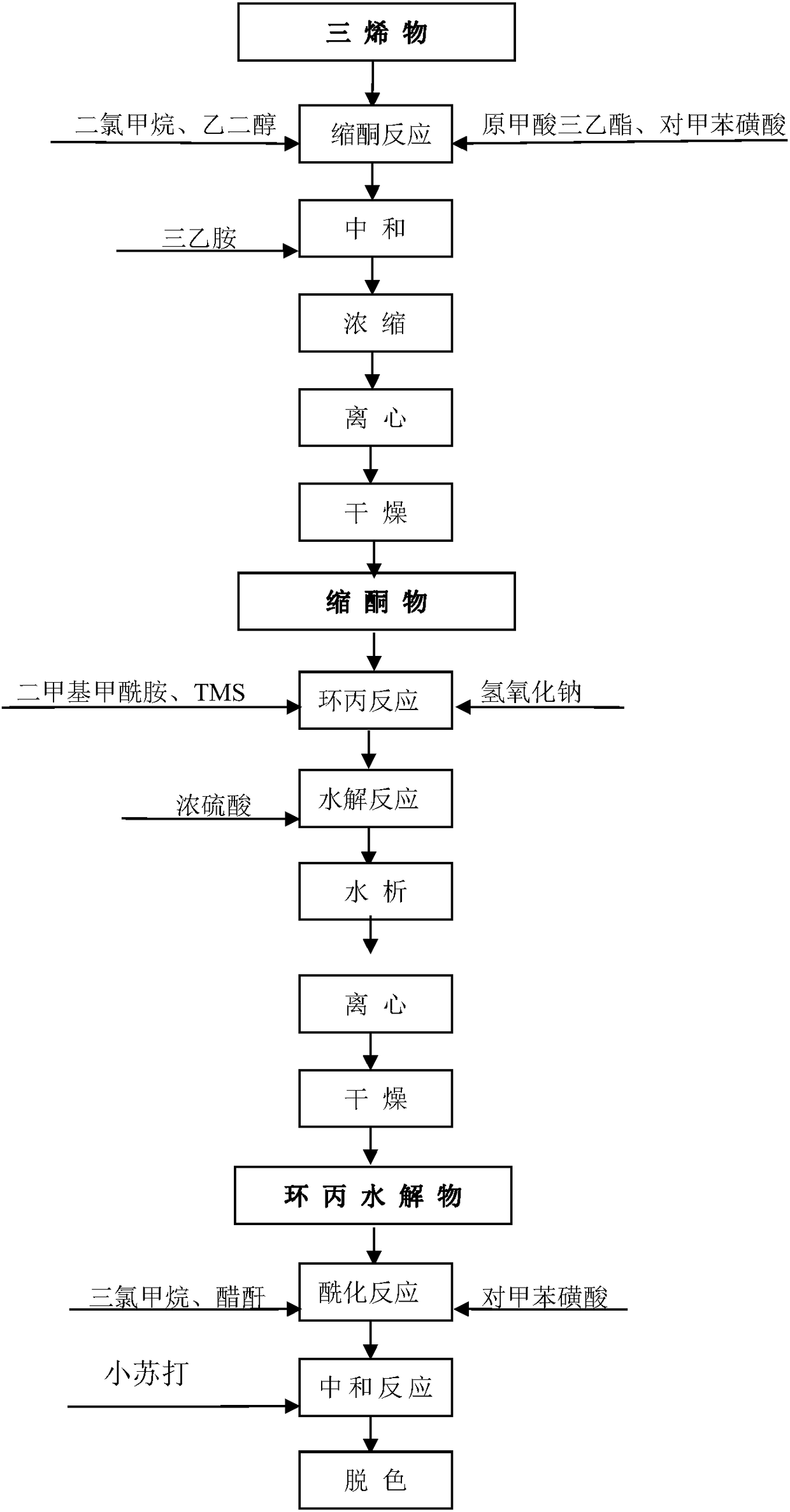
[0018] Ketal reaction: pump dichloromethane, ethylene glycol, and triethyl orthoformate into the reaction kettle, add dehydrogenation substance, PTS, and react at a temperature of 30°C for 5 hours, add triethylamine to neutralize the pH to 8, negative Concentrate under pressure to recover dichloromethane, add methanol, freeze and crystallize until thick, centrifuge, and dry to obtain the ketal product;
[0019] Cyclopropane hydrolysis reaction: Add DMF, ketal and TBS salt in the reaction kettle, add caustic soda three times under stirring at 42°C, react for 3 hours after addition, add the prepared dilute sulfuric acid to adjust the pH value to 2 and hydrolyze for 2 hours After the hydrolysis, the material is pumped to the water analysis tank for water analysis, centrifuged, washed with water until neutral, and dried to obtain cyclopropane hydrolyzate;
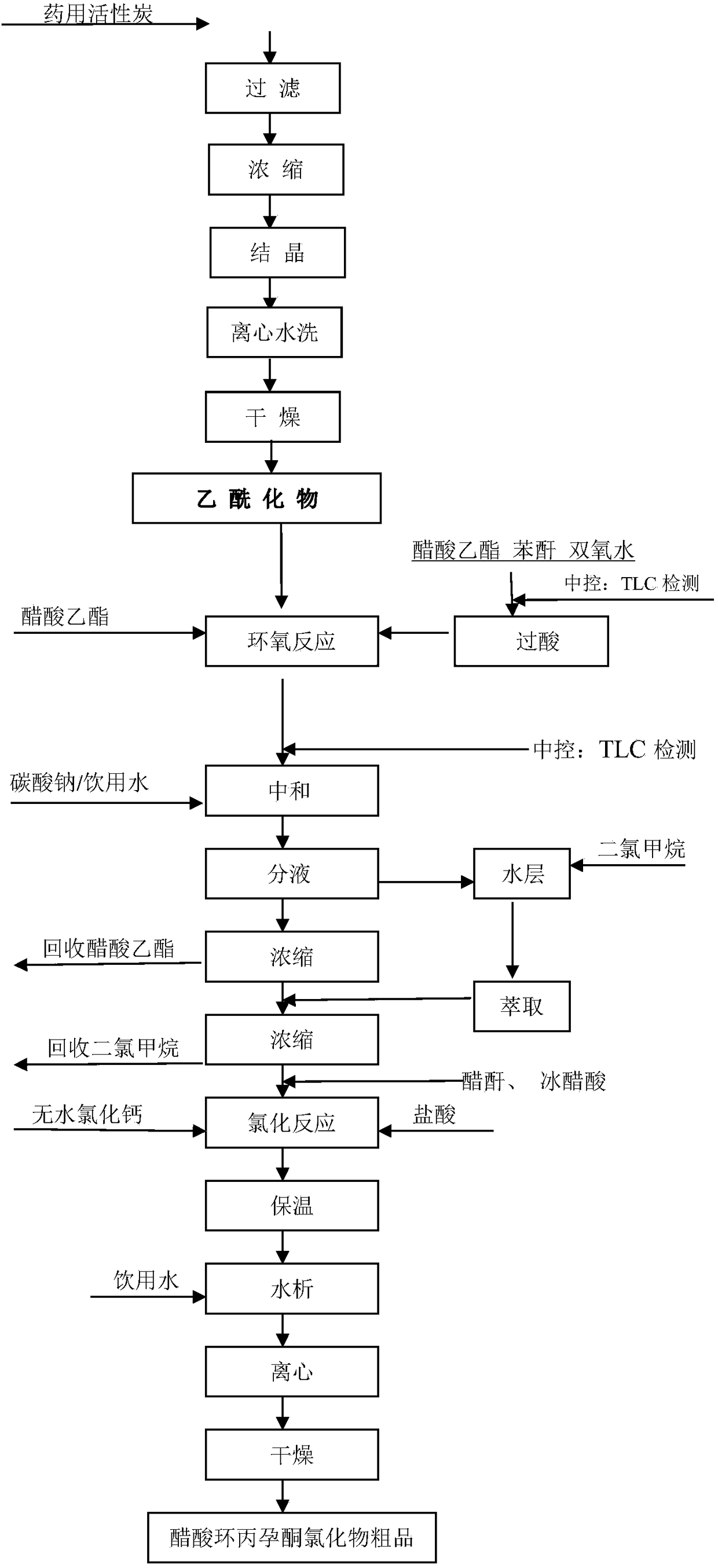
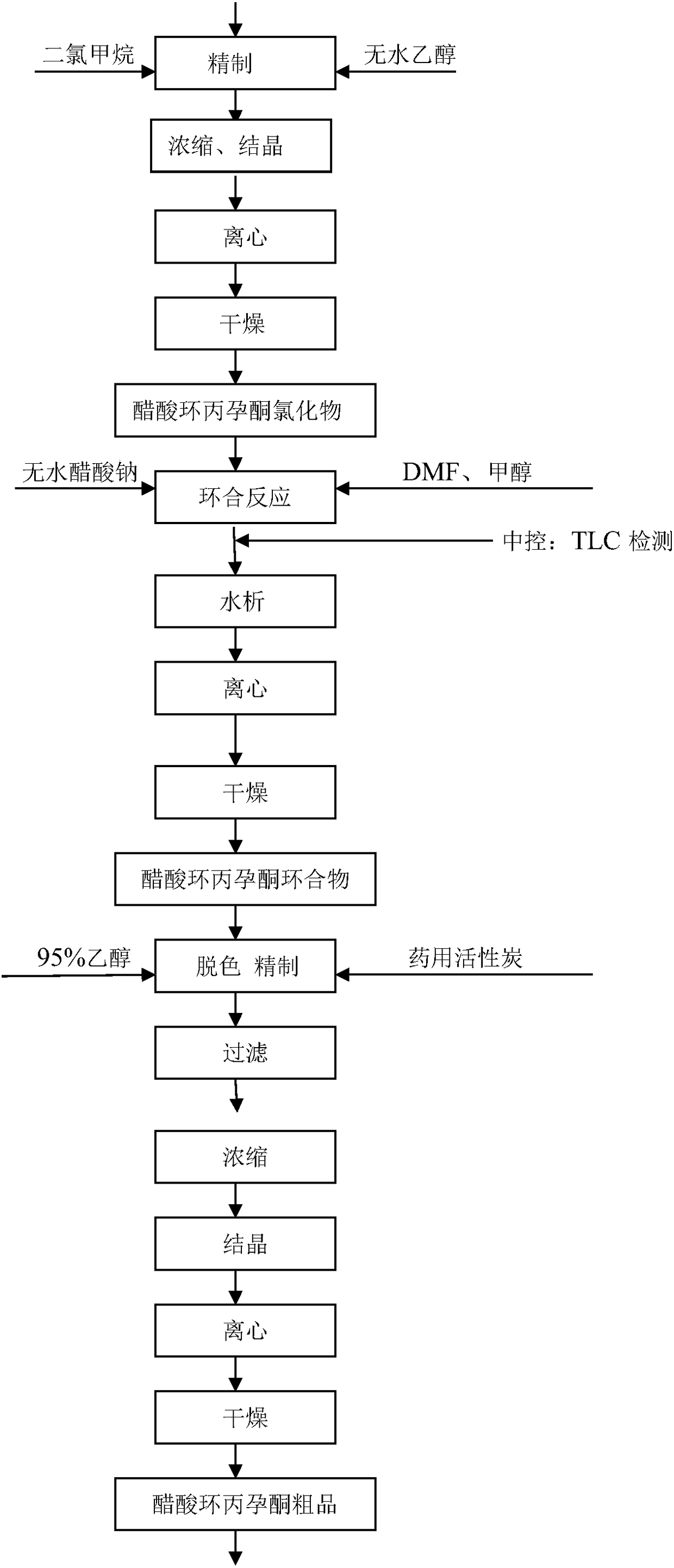
[0020] Acetyl reaction: pump chloroform and acetic anhydride into the reactor, add cyclopropane hydrolyzate and PTS, heat up ...
Embodiment 2
[0026] Ketal reaction: pump dichloromethane, ethylene glycol, and triethyl orthoformate into the reaction kettle, add dehydrogenate, PTS, react at a temperature of 31°C for 5 hours, add triethylamine to neutralize the pH to 8, negative Concentrate under pressure to recover dichloromethane, add methanol, freeze and crystallize until thick, centrifuge, and dry to obtain the ketal product;
[0027] Cyclopropane hydrolysis reaction: Add DMF, ketal and TBS salt in the reaction kettle, add caustic soda three times under stirring at 43°C, react for 3 hours after addition, add the prepared dilute sulfuric acid to adjust the pH value to 2 and hydrolyze for 2 hours After the hydrolysis, the material is pumped to the water analysis tank for water analysis, centrifuged, washed with water until neutral, and dried to obtain cyclopropane hydrolyzate;
[0028] Acetyl reaction: pump chloroform and acetic anhydride into the reaction kettle, add cyclopropane hydrolyzate and PTS, heat up to 59°C ...
Embodiment 3
[0034] Ketal reaction: pump dichloromethane, ethylene glycol, and triethyl orthoformate into the reaction kettle, add dehydrogenate, PTS, and react at a temperature of 32°C for 5 hours, add triethylamine to neutralize the pH to 8, negative Concentrate under pressure to recover dichloromethane, add methanol, freeze and crystallize until thick, centrifuge, and dry to obtain the ketal product;
[0035] Cyclopropane hydrolysis reaction: Add DMF, ketal and TBS salt into the reaction kettle, add caustic soda three times under stirring at 45°C, add reaction for 3 hours, add the prepared dilute sulfuric acid to adjust the pH value to 2 and hydrolyze for 2 hours After the hydrolysis, the material is pumped to the water analysis tank for water analysis, centrifuged, washed with water until neutral, and dried to obtain cyclopropane hydrolyzate;
[0036] Acetyl reaction: pump chloroform and acetic anhydride into the reaction kettle, add cyclopropane hydrolyzate and PTS, heat up to 60°C an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com