Synthesis production method of bromhexini hydrochloride
A technology of bromhexine hydrochloride and production method, which is applied in the field of drug synthesis, can solve the problems of unstable performance of intermediates, rare starting materials, and low product yield, and achieve easy control, easy availability of starting materials, and product yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 240g of water and 100g of 30% hydrochloric acid into the four-necked reaction flask, cool to below 0-5°C,
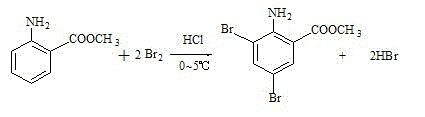
[0029] Add 160 g of bromine dropwise at 0-5°C (1 hour to complete the drop), after the drop is complete, stir and keep warm for 30 minutes to obtain hypobromous acid for later use. in another Add 440g of water into the reaction bottle, start stirring, and drop into methyl anthranilate 60 g, after cooling to 0--5°C. Slowly add hypobromous acid dropwise, control the temperature below 10°C, drop it in about 2 hours, continue to keep warm and stir for 1 hour, add 3000g of water, stir for 30 minutes, discharge the material and suction filter, rinse the filter cake with a large amount of drinking water, To neutrality, discharge and dry. 118g of the product is obtained. It is off-white or light yellow powder, with a melting point of 80~87°C. See figure 2 .
[0030]
[0031] Embodiment 2: the preparation of reduction
Embodiment 2
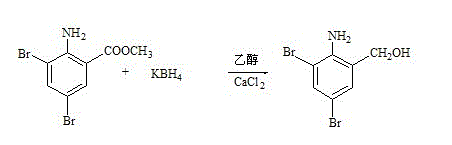
[0032] Put 200g of ethanol and 50g of calcium chloride into the reaction bottle under stirring, and stir until completely dissolved. Put 200g of ethanol into another reaction bottle, add 100g of bromide, 35g of potassium borohydride, stir at room temperature for 30 minutes, and start to add chlorine Calcium chloride ethanol solution, control the reaction temperature at 40±2°C, drop it after 2 hours, keep it warm for 2 hours. Cool down to 25°C, control the temperature below 25°C, slowly add 200 kg of 15% hydrochloric acid, adjust the pH value to 1-2, stir for 30 minutes to maintain the pH value, cool, filter with suction, and rinse the filter cake with drinking water until Neutral, tumble dry. The reduced product was 89g, white or light yellow powder, with a melting point of 135~143°C. See image 3 .
[0033]
[0034] Embodiment 3: Preparation of bromhexine hydrochloride crude product (condensation, salt formation)
Embodiment 3
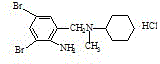
[0035] Put 89g of reducing substance and 135.g of N-methylcyclohexylamine into the reaction flask in sequence, and start stirring for 30 minutes. The temperature is raised to reflux, and after reflux occurs, the water is separated by distillation until the water separation is completed. Continue to keep the reflux reaction for 2 hours, slowly raise the temperature to 170° C., and cool naturally. The temperature was lowered to 50°C, and 100 g of ethanol was sucked in. Then add 160 g of 15% hydrochloric acid until the pH reaches 1-2, and keep the pH constant for 30 minutes. Blowing and suction filtration, the filter cake was washed with 40 g of ethanol, drained, and dried. 100 g of crude bromhexine hydrochloride was obtained, with a melting point of 235-243°C. See Figure 4 .
[0036]
[0037] Embodiment 4: the refining of bromhexine hydrochloride
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com