Synthesis method of 2, 2-diaryl acetonitrile compound
A compound and diaryl technology, applied in the field of synthesis of 2,2-diarylacetonitrile compounds, can solve the problems of harsh reaction conditions, cumbersome reaction operations, complex catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
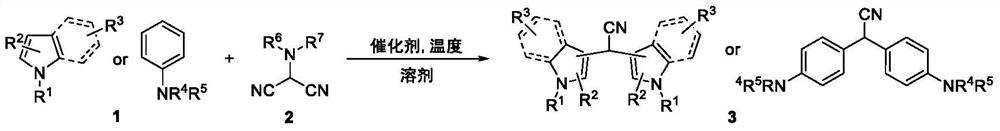
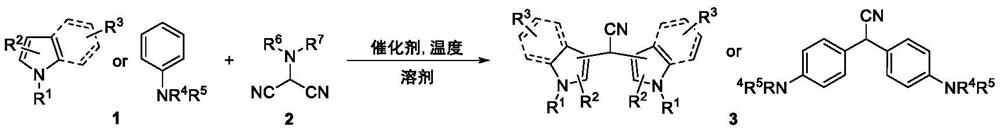
[0021] 1. Use indole and N,N-dimethylaminomalononitrile as raw materials, and aluminum trifluoromethanesulfonate as a catalyst (reaction formula 1)
[0022]
[0023] In a pressure-resistant sealed tube, add indole (100mg, 0.85mmol), N,N-dimethylaminomalononitrile (112mg, 1.02mmol), three Aluminum fluoromethanesulfonate (81.06 mg, 0.17 mmol), under an argon atmosphere, the mixture was continuously stirred and reacted at 120° C. for 8 hours, and the reaction was completed. The reaction tube was washed with acetone and the resulting organic compound system was evaporated to dryness on a rotary evaporator and purified by column chromatography to give pure desired product as a white solid (103.06 mg, 89%).
[0024] The product detection data are as follows:
[0025] 1 H NMR (600MHz, DMSO-d 6 )δ11.17(s,2H),7.59(d,J=7.8Hz,2H),7.43–7.41 (m,4H),7.12(t,J=7.8Hz,2H),7.00(t,J=7.8 Hz,2H),6.08(s,1H); 13 C NMR (150MHz, DMSO-d 6 )δ136.54, 125.20, 123.69, 121.52, 120.68, 118.87, 118.49...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com