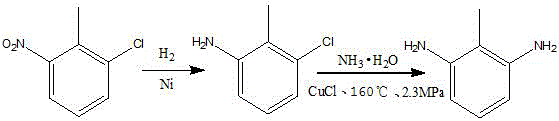
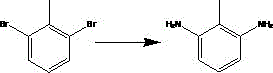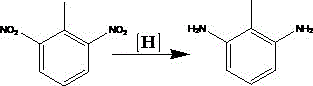Preparation method of 2,6-diaminotoluene
A technology for diaminotoluene and dichlorotoluene, which is applied in the field of catalytic ammoniation to prepare 2,6-diaminotoluene, can solve the problems of difficult industrialization, complex and harsh reaction conditions, and difficult to obtain raw materials, and achieves short chemical paths and reduced The effect of hidden safety hazards and obvious cost advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 palladium ligand catalyst
[0040] A) In a two-necked flask equipped with magnetic stirring, add 250mL of distilled water, and pass through argon for 30 minutes;
[0041] B) Continue to maintain the argon flow, and add PdCl in sequence 2 (10mmol, 1.77g, 1 equiv) and KCl (20mmol, 1.42g, 2equiv), then seal the mouth of the flask with a rubber stopper, and stir the mixture in the bottle for 1 hour;
[0042] C) Inject excess cinnamyl chloride (Ph-allyl)Cl (30mmol, 4.58g, 3equiv) into the reaction flask through a rubber stopper, and the resulting system continued to react for 18 hours;
[0043] D) The reacted mixture was extracted 3 times with chloroform, the organic phases were combined, anhydrous MgSO 4 Drying, filtration, and desolventization under reduced pressure to obtain the corresponding dimer;
[0044] E) Put the ligand N-[2-bis(1-adamantyl)phosphophenyl]morpholine (2.2mmol, 1.02g) and 15mL dry tetrahydrofuran (THF) into a single-n...
Embodiment 2
[0046] The preparation of embodiment 2 palladium ligand catalyst
[0047] A) In a two-necked flask equipped with magnetic stirring, add 250mL of distilled water, and pass through argon for 30 minutes;
[0048] B) Continue to maintain the argon flow, and add PdCl in sequence 2 (10mmol, 1.77g, 1 equiv) and KCl (2.84g, 4 equiv), then seal the flask mouth with a rubber stopper, and stir the mixture in the bottle for 1 hour;
[0049] C) Inject excess cinnamyl chloride (Ph-allyl)Cl (4.58g, 3equiv) into the reaction flask through a rubber stopper, and the resulting system continued to react for 24 hours;
[0050] D) The reacted mixture was extracted 3 times with chloroform, the organic phases were combined, anhydrous MgSO 4 Drying, filtration, and desolventization under reduced pressure to obtain the corresponding dimer;
[0051] E) Put the ligand N-[2-bis(1-adamantyl)phosphophenyl]morpholine (2.2mmol, 1.02g) and 15mL dry tetrahydrofuran (THF) into a single-necked flask equipped w...
Embodiment 3
[0054] In a 1500mL reaction flask, add 500g of absolute ethanol, 100g of 2,6-dichlorotoluene, 300g of ammonia water and 6g of palladium complex catalyst, heat and start stirring under normal pressure, raise the temperature to 100°C, and keep it warm for 10 hours . After the reaction was completed, the catalyst was filtered off, the ethanol was evaporated, and 200 g of distilled water was added, the system was cooled to room temperature, filtered, and dried to obtain 57.4 g of 2,6-diaminotoluene.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com