Preparation method and detection method for edaravone dimer and tautomer thereof
A technology of edaravone dimers and tautomers, applied in the field of edaravone dimers, can solve the problems of potential safety hazards in clinical use of drugs, poor solubility of dimers, etc., and achieve safety assurance, The effect of easy operation and easy availability of starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
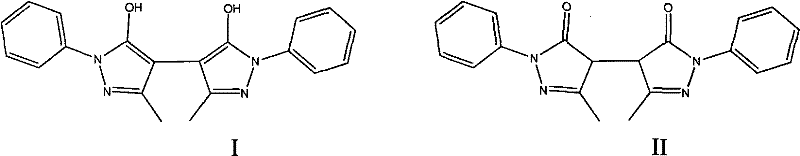
[0020] Embodiment 1: the preparation of Edaravone dimer
[0021] Weigh 5.0g of Edaravone and 3.0g of sodium bisulfite, dissolve them in 250ml of water, heat to about 100°C, keep stirring for 24 hours, a red solid gradually precipitates in the reaction solution, cool to room temperature, filter with ordinary filter paper, the solid is Wash with 5ml each of water, methanol, and ethyl acetate, and dry in vacuo at 60°C to obtain 0.2g of light brown powder.
[0022] Structural confirmation: MS (ESI): 347[M+H]+, 369[M+Na]+
[0023] 1H NMR: 11.49, s, 2H (5-OH, 5'-OH); 7.77, m, 4H (11-H, 7-H, 11'-H, 7'-H, J=7.6); 7.46, t, 4H (10-H, 8-H, 10'-H, 8'-H, J=7.6); 7.23, t, 2H (9-H, 9'-H, J=7.6); 2.20, s , 6H(3-CH3, 3'-CH3)
Embodiment 2
[0024] Embodiment 2: the preparation of Edaravone dimer
[0025] Weigh 5.0g of Edaravone and 0.5g of sodium bisulfite, suspend in 500ml of methanol, heat to about 65°C, keep stirring for 72h, a red solid gradually precipitates in the reaction solution, cool to room temperature, filter, and dissolve the solid with water , methanol, and ethyl acetate were washed separately, and after drying, 0.05 g of light brown powder was obtained.
[0026] Structure confirmation: MS (ESI) and 1H NMR data are the same as those in Example 1.
Embodiment 3
[0027] Embodiment 3: the preparation of Edaravone dimer
[0028] Weigh 5.0g of Edaravone and 3.5g of sodium thiosulfate, suspend in 200ml of ethanol, heat to about 80°C, keep stirring for 48h, a red solid gradually precipitates in the reaction solution, cool to room temperature, filter, and dissolve the solid with ethanol , water, methanol, and ethyl acetate were washed with 5ml each, and after vacuum drying, 0.13g of a light brown powder was obtained.
[0029] Structure confirmation: MS (ESI) and 1H NMR data are the same as those in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com