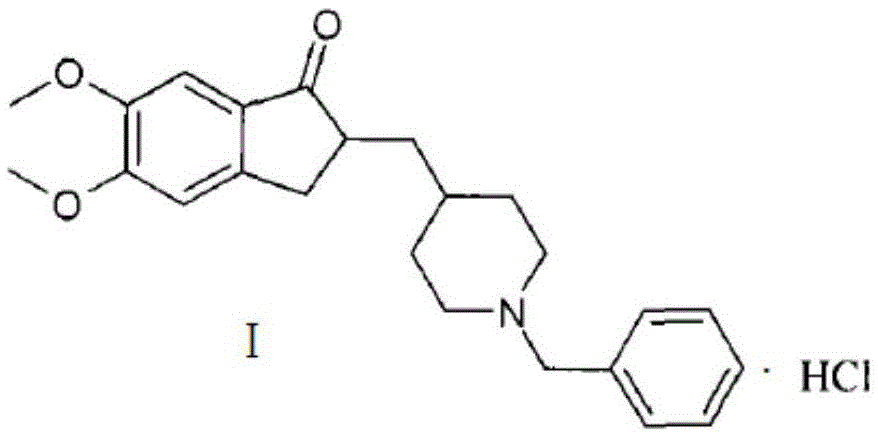
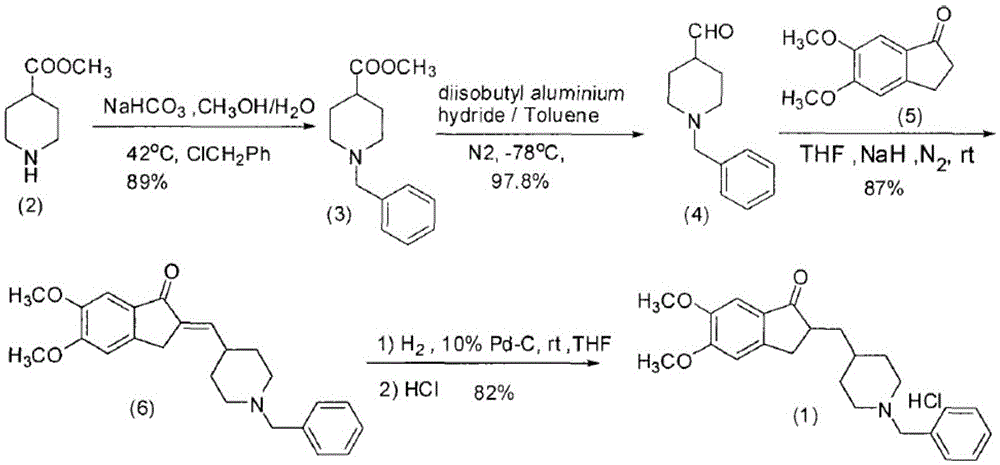
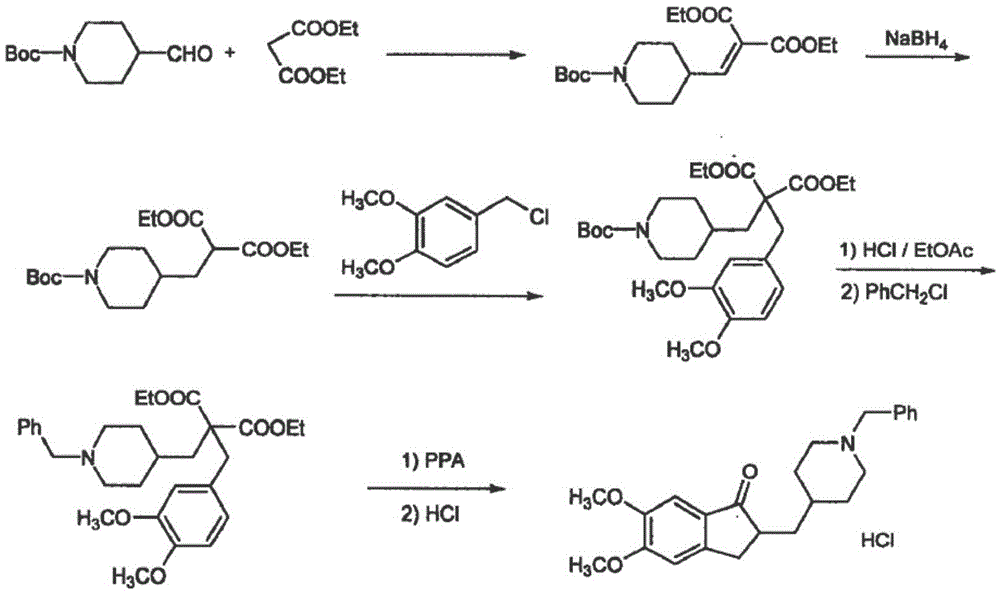
Preparation method of donepezil hydrochloride
A technology for forming donepezil hydrochloride and salt formation is applied in the field of preparation of donepezil hydrochloride, which can solve the problems of many steps, low yield and the like, and achieves the effects of mild reaction conditions, high yield and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Using 1-ethyl-3-methylimidazole-aluminum chloride as the ionic liquid catalytic system, the specific steps are as follows:
[0029] [Emim]Cl-AlCl 3 Preparation of (Emim=1-ethyl-3-methylimidazole)
[0030] A stirrer was installed on the reaction kettle, and 26.67g (0.2mol) of anhydrous aluminum trichloride was added under nitrogen protection, and 29.3g (0.2mol) of 1-ethyl 3-methylimidazole chloride was added in batches, and kept Stir at around 50°C for 3 hours to ensure complete reaction and produce transparent light yellow [Emim]Cl-AlCl 3 ionic liquid.
[0031] Preparation of 1-benzyl-4-(5,6-dimethoxy-1-indanone-2-methylene)-piperidine (Ⅳ)
[0032] Under nitrogen protection, 22.9 g (0.1 mol) of 3-chloro-1-(3,4-dimethoxyphenyl) propan-1-one (II), N-benzyl-4 -Piperidinyl formaldehyde (Ⅲ) 20.3g (0.1mol), [Emim]Cl-AlCl 3 (0.2mol), control the temperature at 40-45°C and maintain the time for 4.5 hours, raise the temperature to 145-155°C and maintain the reaction for 5.5...
Embodiment 2
[0035] Preparation of ionic liquids
[0036] Install a stirrer on the reactor, add 27.3g (0.2mol) of anhydrous ZnCl under nitrogen protection 2 , 34.9 g (0.2 mol) of 1-butyl-3-methylimidazole chloride ([Bmim]Cl) was added in portions. After adding the imidazolium salt, keep stirring at about 40°C for 3 hours to ensure that the reaction is complete, and a colorless and transparent [Bmim]Cl-ZnCl is obtained 2 ionic liquid.
[0037] Preparation of 1-benzyl-4-(5,6-dimethoxy-1-indanone-2-methylene)-piperidine (Ⅳ)
[0038] Under nitrogen protection, 22.9 g (0.1 mol) of 3-chloro-1-(3,4-dimethoxyphenyl) propan-1-one (II), N-benzyl-4 -Piperidinyl formaldehyde (Ⅲ) 20.3g (0.1mol), [Bmim]Cl-ZnCl 2 (0.2mol), control the temperature at 45-50°C and maintain it for 4 hours, raise the temperature to 155-165°C and maintain the reaction for 5 hours, monitor the end point of the reaction by TLC, after the reaction, cool down to room temperature, the product and the ionic liquid are automatica...
Embodiment 3
[0040] Preparation of ionic liquids
[0041] Install a stirrer on the reaction kettle, add 40.0g (0.3mol) of anhydrous AlCl under nitrogen protection 3 , add 27.5g (0.2mol) triethylamine hydrochloride ([Et 3 NH]Cl). After adding triethylamine hydrochloride, keep stirring at about 60°C for 3 hours to ensure complete reaction and obtain transparent [Et 3 NH]Cl-AlCl 3 ionic liquid.
[0042] Preparation of 1-benzyl-4-(5,6-dimethoxy-1-indanone-2-methylene)-piperidine (Ⅳ)
[0043] Under nitrogen protection, 22.9 g (0.10 mol) of 3-chloro-1-(3,4-dimethoxyphenyl) propan-1-one (II), N-benzyl-4 -Piperidinyl formaldehyde (Ⅲ) 22.4g (0.11mol), [Et 3 NH]Cl-AlCl 3 (2:3, by AlCl 3 Calculated as 0.3mol), control the temperature at 55-60°C and maintain it for 5 hours, raise the temperature to 165-175°C and maintain the reaction for 6 hours, TLC monitors the end point of the reaction, after the reaction, cool down to room temperature, the product and the ionic liquid are automatically sep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com