Meropenem intermediate and preparation method thereof
A technology of meropenem and intermediates, which is applied in the field of medicine, can solve the problems of unfriendly water body and ecological environment, increase the cost of solid-liquid waste liquid treatment, and the inability to recover phosphorus-containing reagents, and achieves increased treatment costs, short preparation time, and high efficiency. Beneficial to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The embodiment of the present application provides a meropenem intermediate represented by formula I, and the specific preparation method includes:
[0068] According to the following synthesis route, add 50ml of dichloromethane, 10g of azabicyclic compounds represented by formula II, and 3.3g of ethyl chloroformate (1.1eq) into a four-necked flask, and cool down to -25°C; Ethylamine (1.2eq) 3.35g, reacted at -25°C for 0.5h, and the product was ready for use, which was the intermediate of meropenem represented by formula I. Mass spectrometry data of the reaction solution, MS (M+1: 435.13).
[0069] The name of the meropenem intermediate shown in formula I is (4R, 5R, 6S)-3-(ethoxycarboxy)-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo Dio-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid-(4-nitro)benzyl ester.
[0070] .
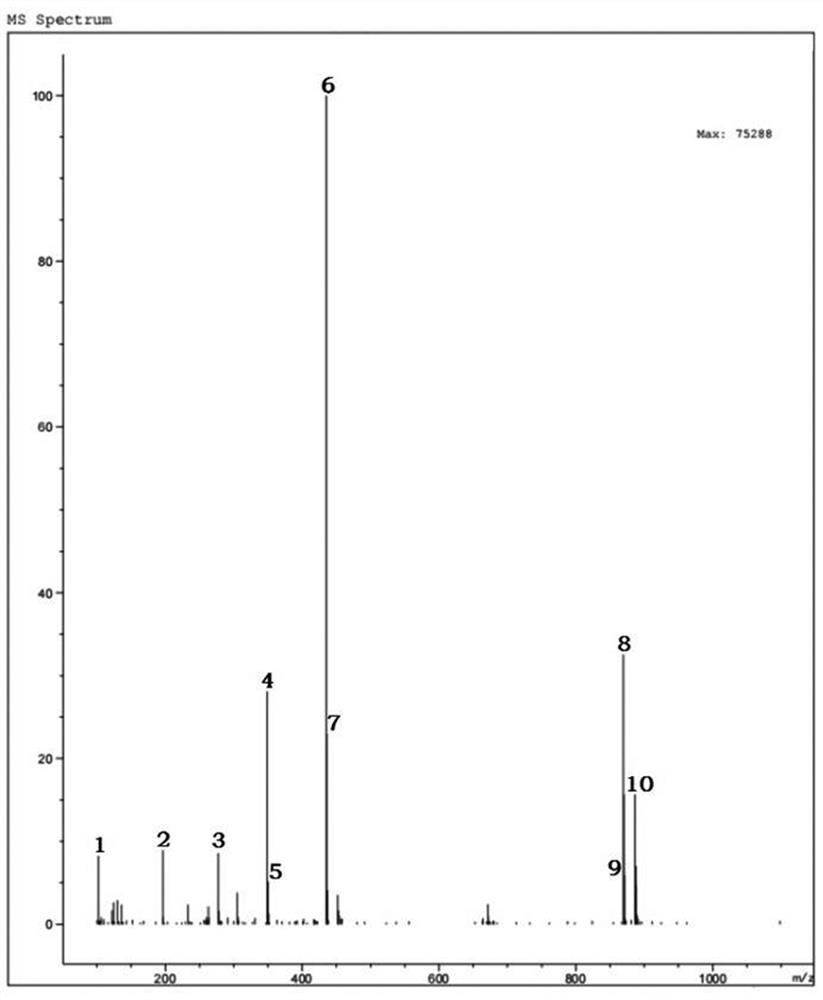
[0071] Detect the mass spectrum of the meropenem intermediate shown in the formula I of the embodiment of the present application, and the results are as f...
Embodiment 2
[0073] The embodiment of the present application provides undeprotected meropenem represented by formula V, and the specific preparation method includes:
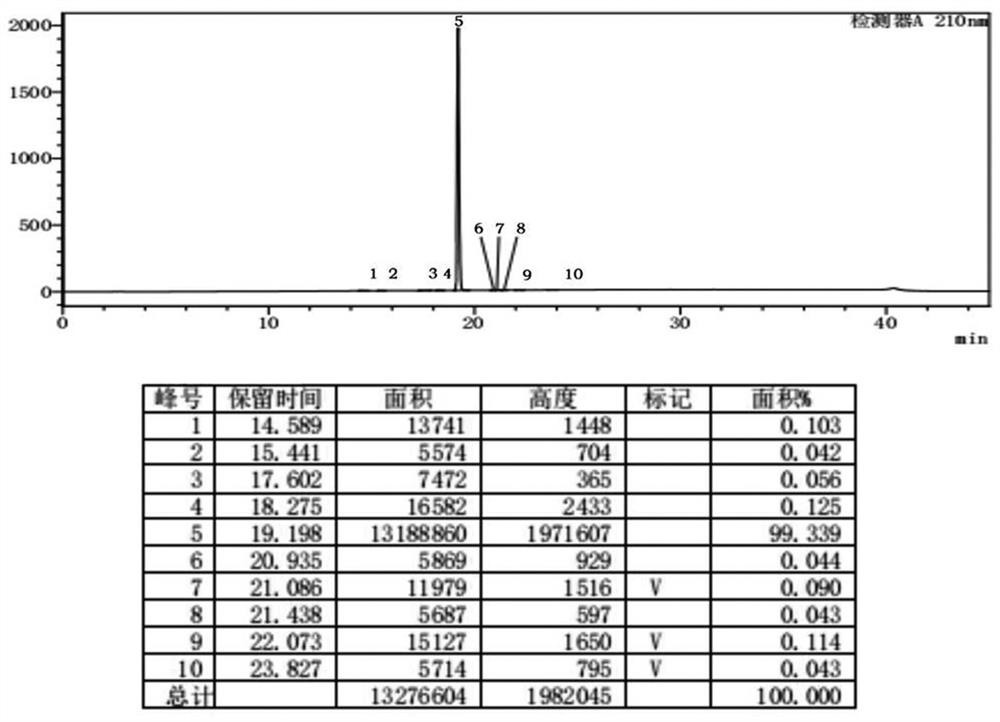
[0074] According to the following synthetic route, add 50ml of acetonitrile and 9.75g of meropenem side chain (1.0eq) shown in formula IV to the product in Example 1, cool down to -15°C; start adding tetramethylguanidine (1.2eq) dropwise 3.82g, reacted at -15°C for 1h. After the reaction was completed, 50 ml of saturated brine was added for washing, and the layers were separated. The aqueous phase was extracted three times with 150 ml of ethyl acetate, and the organic phases were combined. Concentrate the organic phase at 45°C to an oily substance, add 50ml of ethyl acetate and stir to dissolve, then cool down to 0°C and stir for crystallization for 2h, filter, and dry at 35°C to obtain 17.66g of undeprotected meropenem represented by formula V , the molar yield of the two-step method was 92%, and the purity by HPLC was 98...
Embodiment 3
[0078] The embodiment of the present application provides a meropenem intermediate represented by formula I, and the specific preparation method includes:
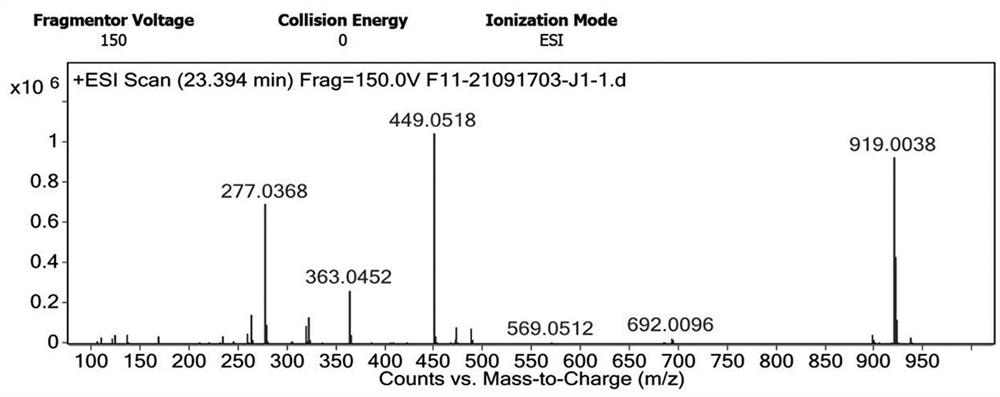
[0079] According to the following synthetic route, add 50ml of dichloromethane, 10g of azabicyclic compounds represented by formula II, and 3.74g of isopropyl chloroformate (1.1eq) into a four-necked flask, and cool down to -20°C; start to add dropwise Triethylamine (1.2eq) 3.35g, reacted at -20°C for 0.5h, and the product was ready for use, which was the intermediate of meropenem represented by formula I. Mass spectrometry data of the reaction solution, MS (M+1: 449.15).
[0080] The meropenem intermediate represented by formula I is (4R, 5R, 6S)-3-(isopropyloxycarboxy)-6-((R)-1-hydroxyethyl)-4-methyl-7- Oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid-(4-nitro)benzyl ester.
[0081] .
[0082] Detect the mass spectrum of the meropenem intermediate shown in the formula I of the embodiment of the present applicatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com