Synthetic method of tropicamide
A synthetic method, the technology of tropic amide, applied in the direction of organic chemistry, etc., can solve the problems of poor stability of intermediate products, difficult promotion and application, and increased cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
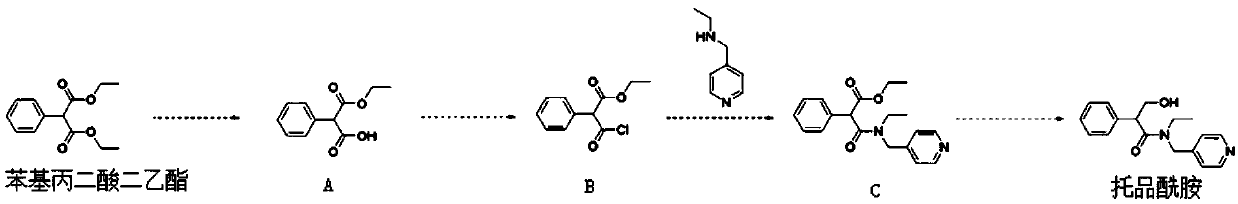
[0059] 1) Using 10mol of diethyl phenylmalonate as raw material, hydrolysis reaction occurs in an inorganic alkali solvent to obtain intermediate product A, wherein the pH of the hydrolysis reaction is 8, the temperature is 0°C, and the time is 0.5h. The molar ratio of diethyl malonate and inorganic base is 1:2;
[0060] 2) intermediate product A and sulfur oxychloride carry out acylation reaction in methylene chloride solvent, obtain intermediate product B, the mol ratio of intermediate product A and sulfur oxychloride is 1:2, and the temperature of described acylation reaction is 0°C, the time is 2h;
[0061] 3) Intermediate product B and 20mol N-ethyl-4-methylpyridinamine undergo condensation reaction in tetrahydrofuran solvent to obtain intermediate product C, the molar ratio of intermediate product B to N-ethyl-4-methylpyridinamine is 1:1.5, the temperature of the condensation reaction is 5°C, and the time is 1h;
[0062] 4) The intermediate product C and 40 mol of calc...
Embodiment 2
[0064] 1) Using 10mol of diethyl phenylmalonate as a raw material, hydrolysis reaction occurs in an inorganic alkali solvent to obtain intermediate product A, wherein the pH of the hydrolysis reaction is 12, the temperature is 34 ° C, and the time is 8 hours. The mol ratio of diethyl diacid and inorganic base is 1:8;
[0065] 2) intermediate product A and sulfur oxychloride carry out acylation reaction in methylene chloride solvent, obtain intermediate product B, the mol ratio of intermediate product A and sulfur oxychloride is 1:20, the temperature of described acylation reaction is 60℃, the time is 22h;
[0066] 3) Intermediate product B and 100mol N-ethyl-4-picoline amine undergo condensation reaction in tetrahydrofuran solvent to obtain intermediate product C, the molar ratio of intermediate product B to N-ethyl-4-picoline amine is 1:6, the temperature of the condensation reaction is 20°C, and the time is 20h;
[0067] 4) The intermediate product C and 300 mol of calcium...
Embodiment 3
[0069] 1) Using 10mol of diethyl phenylmalonate as a raw material, hydrolysis reaction occurs in an inorganic alkali solvent to obtain intermediate product A, wherein the pH of the hydrolysis reaction is 10, the temperature is 18°C, and the time is 4h. The mol ratio of diethyl diacid and inorganic base is 1:5;
[0070] 2) intermediate product A and sulfur oxychloride carry out acylation reaction in methylene chloride solvent, obtain intermediate product B, the mol ratio of intermediate product A and sulfur oxychloride is 1:11, the temperature of described acylation reaction is 30℃, the time is 12h;
[0071] 3) Intermediate product B and 60mol N-ethyl-4-methylpyridinamine undergo condensation reaction in tetrahydrofuran solvent to obtain intermediate product C, the molar ratio of intermediate product B to N-ethyl-4-methylpyridinamine is 1:3.5, the temperature of the condensation reaction is 12°C, and the time is 10h;
[0072] 4) The intermediate product C and 170 mol of potas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com