Improved synthetic method of penoxsulam
A penoxsulam and synthetic method technology, applied in the direction of organic chemistry, can solve the problems of unsatisfactory results, increased content of impurities in by-products, unfavorable products, etc., and achieve safe post-processing steps, less product loss, The effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
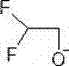
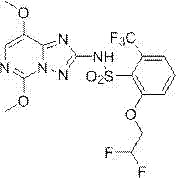
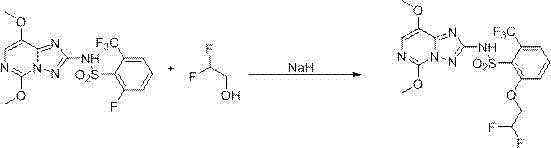
[0040]The synthesis method of penoxsulam described in the present invention specifically relates to the improvement of the etherification step of preparing penoxsulam through etherification of tetrafluorosulfonamide. The etherification step uses strong basic substances, difluoro Ethanol and tetrafluorosulfonamide (an intermediate in the synthesis of penoxsulam, 2-fluoro-6-trifluoromethyl-N-(5,8-dimethoxy-[1,2,4]triazolo [1,5-c]pyrimidine-2)-benzenesulfonamide for short) as raw material, the specific synthesis process includes the following steps:
[0041] I, the preparation of A liquid
[0042] Under the protection of nitrogen, firstly, the strongly basic substance was dissolved in ethylene glycol dimethyl ether under ice-water bath conditions to obtain a strongly basic solution; then, difluoroethanol was added dropwise to the strongly basic solution, and passed Control the rate of addition of difluoroethanol to control the temperature of the reaction system below 10°C to pre...
example 1
[0056] In a 2000mL four-neck round bottom flask equipped with a thermometer, nitrogen balloon and rubber stopper, add 700g of ethylene glycol dimethyl ether, under the protection of nitrogen, cut 30.4g of peeled metal sodium (1.32mol, 2.2eq) into small pieces Add the block into the system, fully stir in the ice-water bath and cool down to about 5°C, slowly add 49.2g (0.6 mol, 2.0eq) of difluoroethanol to the system dropwise, pay attention to the obvious heat release during the dropping process, and a large amount of gas is generated, the reaction system The temperature is controlled below 10°C, and the drop rate of difluoroethanol is adjusted by changing the temperature. After the dropwise addition was completed, tetrafluorosulfonamide (126 g, 0.3 mol, 1.0 eq) was slowly added to the system in batches at about 10°C. The addition process is still accompanied by a large amount of gas generation and obvious exothermic phenomenon. Control the addition speed so that the system temp...
example 2
[0059] Potassium tert-butoxide is used instead of sodium metal. For a specific implementation, refer to Example 1. The product is dried, and the HPLC content determination is >98%, and the yield is 93.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com