Synthesis method of mefenpyr-diethyl
A technology for the synthesis of mefenpyr-ethyl and the synthesis method, which is applied in the field of synthesis of mefenpyr-ethyl, can solve the problems of long synthetic route, high cost and low yield, and achieve short reaction route, low cost and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
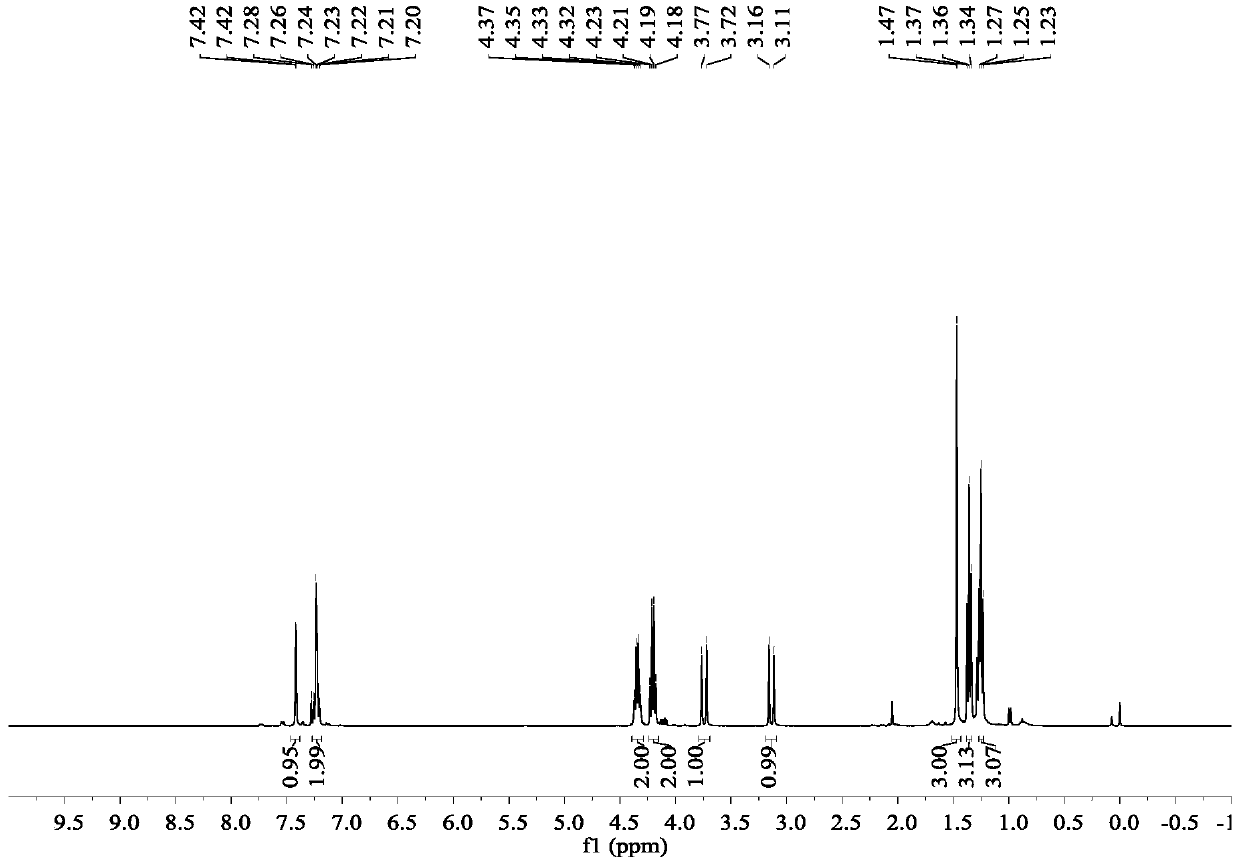
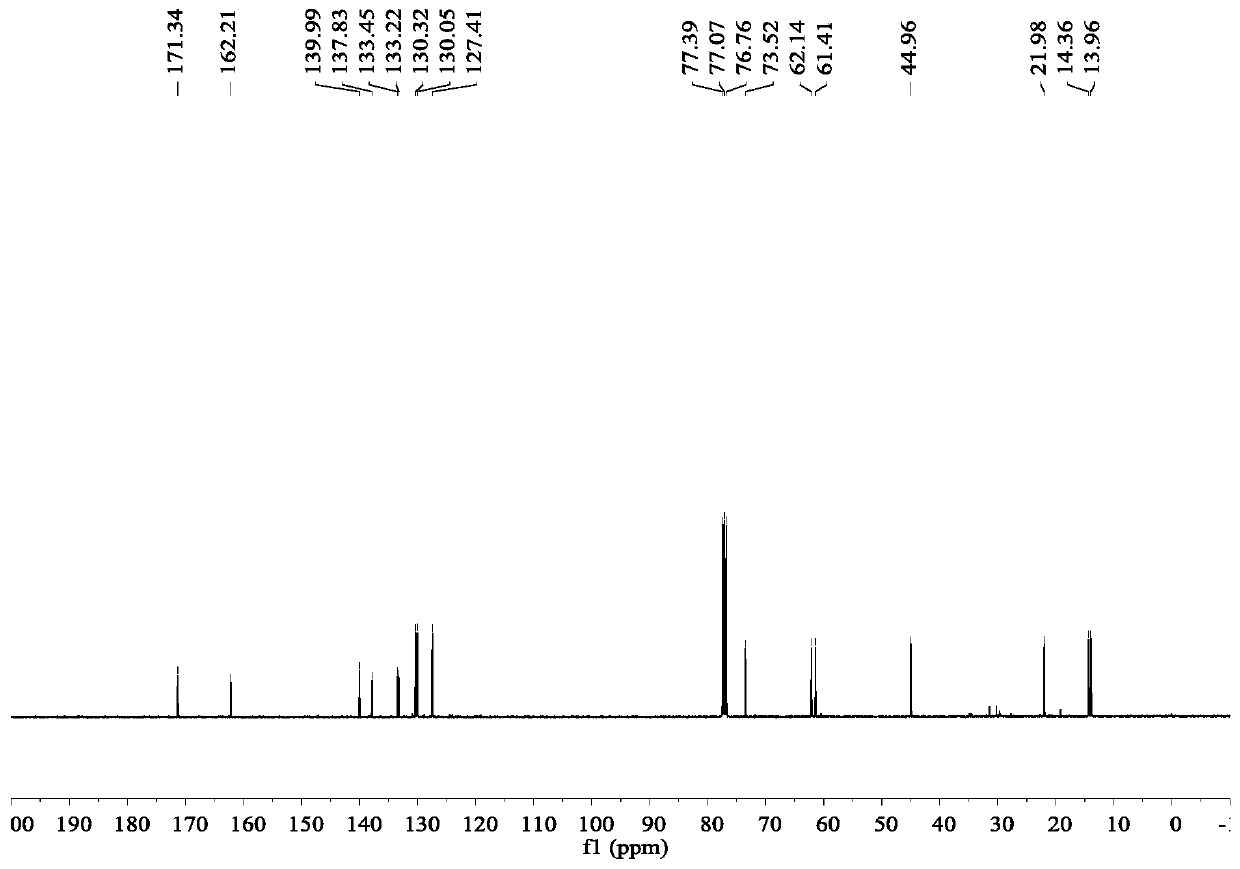
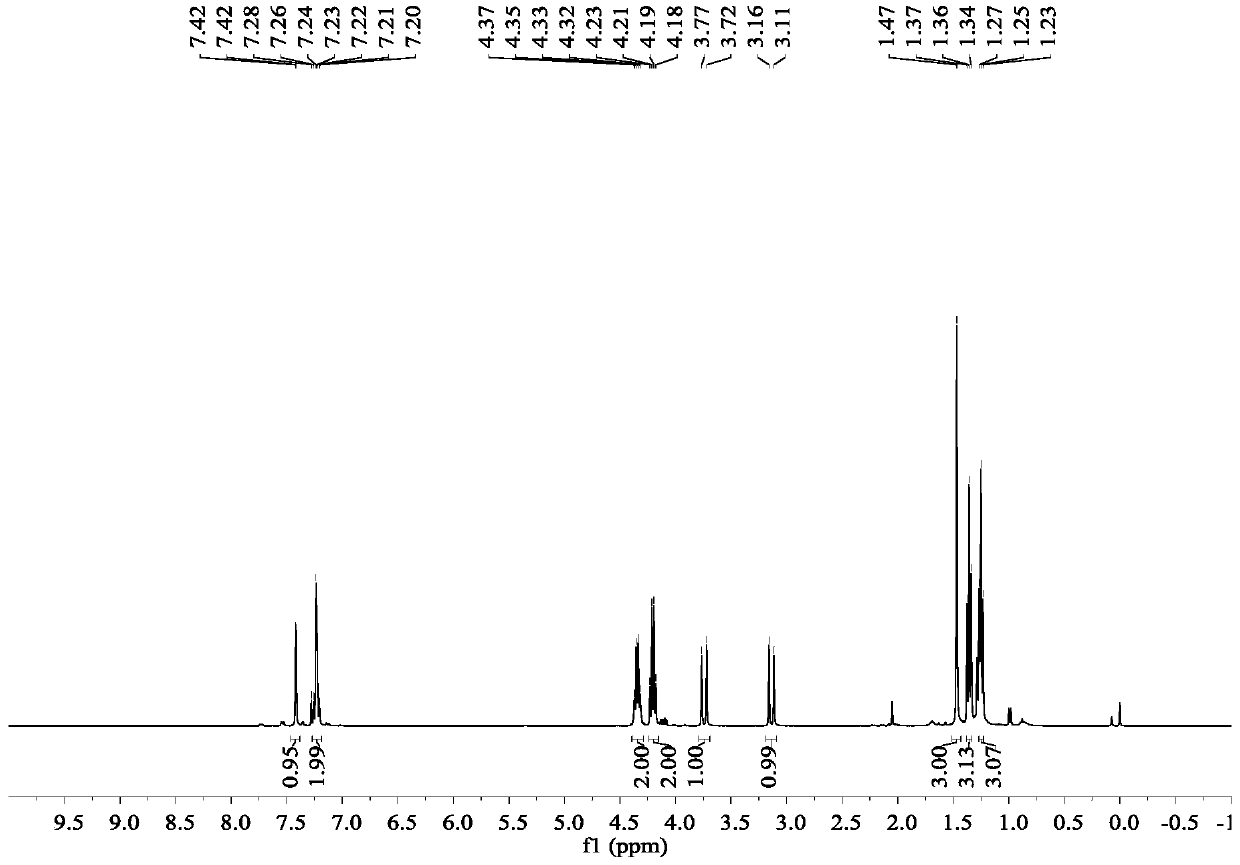
Image
Examples
Embodiment 1
[0040] Synthesis of ethyl 2-[2-(2,4-dichlorophenyl)hydrazinomethylene]acetate:
[0041] 2,4-Dichlorophenylhydrazine hydrochloride (21.3g, 0.1mol) was dissolved in 200ml of ethanol, triethylamine (10.1g, 0.1mol) was added, and 50% ethyl glyoxylate in toluene was added dropwise (40.8g, 0.2mol), the addition was completed in 30 minutes, the temperature was raised to reflux and stirred for 4 hours after the addition, the solvent was evaporated under reduced pressure, and the anhydrous ethanol was recrystallized to obtain 2-[2-(2,4-dichlorophenyl)hydrazino Methyl]ethyl acetate 16.1g, yield: 62%.
Embodiment 2
[0043] Synthesis of ethyl 2-[2-(2,4-dichlorophenyl)hydrazinomethylene]acetate:
[0044]Dissolve 2,4-dichlorophenylhydrazine hydrochloride (21.3g, 0.1mol) in 200ml tetrahydrofuran, add triethylamine (12.1g, 0.12mol), and add dropwise 50% ethyl glyoxylate in toluene (24.4g, 0.12mol), the addition was completed in 30 minutes, after the addition, the temperature was raised to reflux and stirred for 4 hours, the solvent was evaporated under reduced pressure, and recrystallized from absolute ethanol to obtain 2-[2-(2,4-dichlorophenyl)hydrazino Methyl]ethyl acetate 15.1g, yield: 58%.
Embodiment 3
[0046] Synthesis of pyraclofen-ethyl:
[0047] Add ethyl 2-[2-(2,4-dichlorophenyl)hydrazinomethylene]acetate (13.0 g, 0.05 mol) and ethyl methacrylate (6.8 g, 0.06 mol) into a 250 mL eggplant flask , add DMF (100mL) and stir to dissolve, then add molecular iodine (2.5g, 0.01mol) and 70% aqueous solution of TBHP (9.6g, 0.075mol) into the reaction system, react at room temperature for 8h, after the reaction is complete, add 100 ml of 10% aqueous sodium bisulfite. Extract with ethyl acetate (100mL×3), wash the ethyl acetate layer with saturated brine (100mL×2), distill off the solvent from the ethyl acetate layer under reduced pressure to obtain an oily liquid, and recrystallize from petroleum ether to obtain pyraclofen-ethyl 13.2 g, yield rate is 70%, mp: 48~51 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com