Mesosulfuron preparation method
The technology of methyldisulfuron-methyl and the methyldisulfuron-methyl is applied in the field of preparation of methyldisulfuron-methyl, which can solve the problems of low yield and the like, and achieve the effects of improved yield, easy availability of raw materials and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
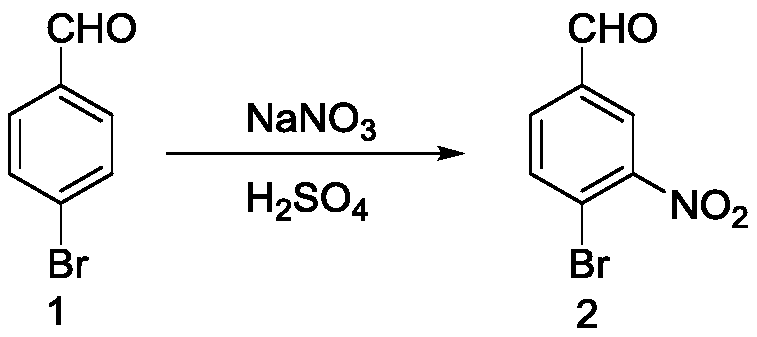
[0063] Preparation of 4-bromo-3-nitrobenzaldehyde (compound 2)
[0064] First add 550mg of sodium nitrate and 6mL of sulfuric acid into a 25mL round-bottomed flask, stir until transparent at room temperature, then slowly add 1g of p-bromobenzaldehyde, and react at room temperature for 1 hour after the addition. After the reaction is over, pour the reaction mixture into Ice water, and then filtered with suction to obtain a milky white solid as compound 2, with a yield of 94%.
[0065] The product data is: 1 H NMR (400 MHz, Chloroform-d) δ 10.03 (d, J=2.0 Hz, 1H), 8.30 (d, J=2.2 Hz, 1H), 7.94 (q, J=2.0 Hz, 2H).
Embodiment 2
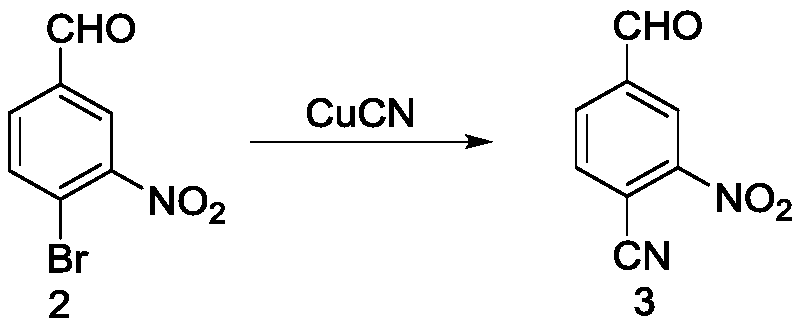
[0067] Preparation of 4-cyano-3-nitrobenzaldehyde (compound 3)
[0068] 1) Add 1g of 4-bromo-3-nitrobenzaldehyde, 429mg of cuprous cyanide and 10mL of DMF into a 50mL round bottom flask, react at 140°C for 1 hour, stop the reaction, filter with suction, and take the filtrate with ethyl acetate After ester extraction, the organic layer was distilled under reduced pressure, and purified by column chromatography to obtain compound 3 with a yield of 71%.
[0069] 2) Add 1g of 4-bromo-3-nitrobenzaldehyde, 585mg of cuprous cyanide and 10mL of DMF into a 50mL round bottom flask, react at 160°C for 1 hour, stop the reaction, filter with suction, and take the filtrate with ethyl acetate After ester extraction, the organic layer was distilled under reduced pressure, and purified by column chromatography to obtain compound 3 with a yield of 76.4%.
[0070] 3) Add 1g of 4-bromo-3-nitrobenzaldehyde, 585mg of cuprous cyanide and 10mL of DMF into a 50mL round bottom flask, react at 140°C fo...
Embodiment 3
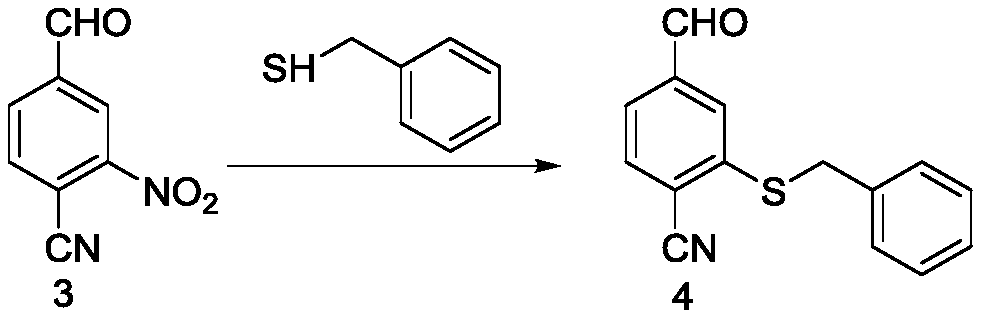
[0074] Preparation of 2-(benzylthio)-4-formylbenzonitrile (compound 4)
[0075] 1) Add 1 g of 4-cyano-3-nitrobenzaldehyde, 739 mg of benzyl mercaptan and 15 mL of DMF into a 50 mL round bottom flask, and cool to 0°C. Dissolve 382mg potassium hydroxide in 1.6ml H 2 An aqueous potassium hydroxide solution was prepared in O, and the potassium hydroxide solution was slowly added dropwise to the above solution under ice-cooling. After the dropwise addition was completed, the mixture was reacted at room temperature for 0.5 hours, and the mixture after the reaction was poured into ice water, and then filtered with suction to obtain a yellow solid as compound 2, which was further purified with a yield of 64.2%.
[0076] 2) Add 1 g of 4-cyano-3-nitrobenzaldehyde, 739 mg of benzyl mercaptan and 15 mL of DMF into a 50 mL round bottom flask, and cool to 0°C. Dissolve 421mg potassium hydroxide in 1.8ml H 2 An aqueous potassium hydroxide solution was prepared in O, and the potassium hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com