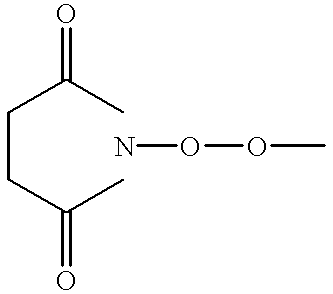
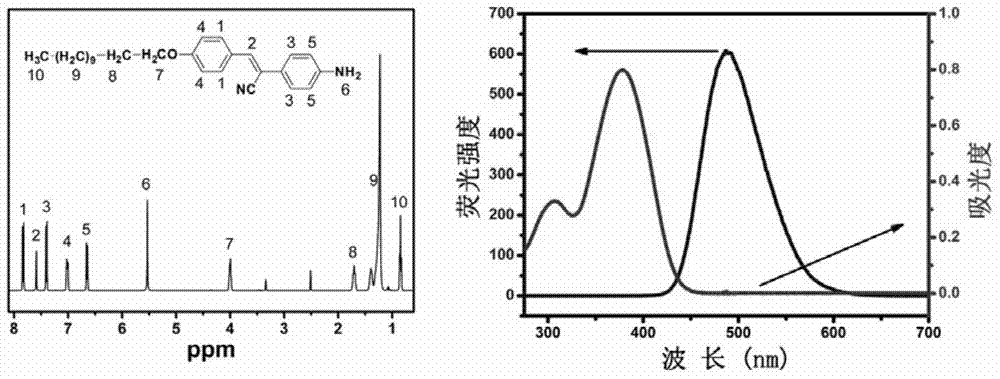
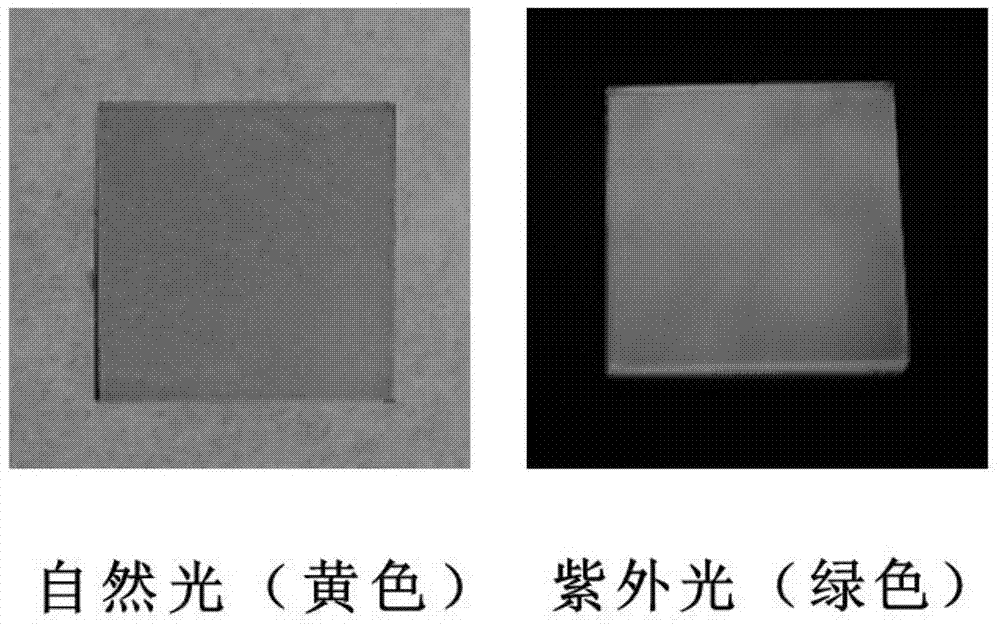
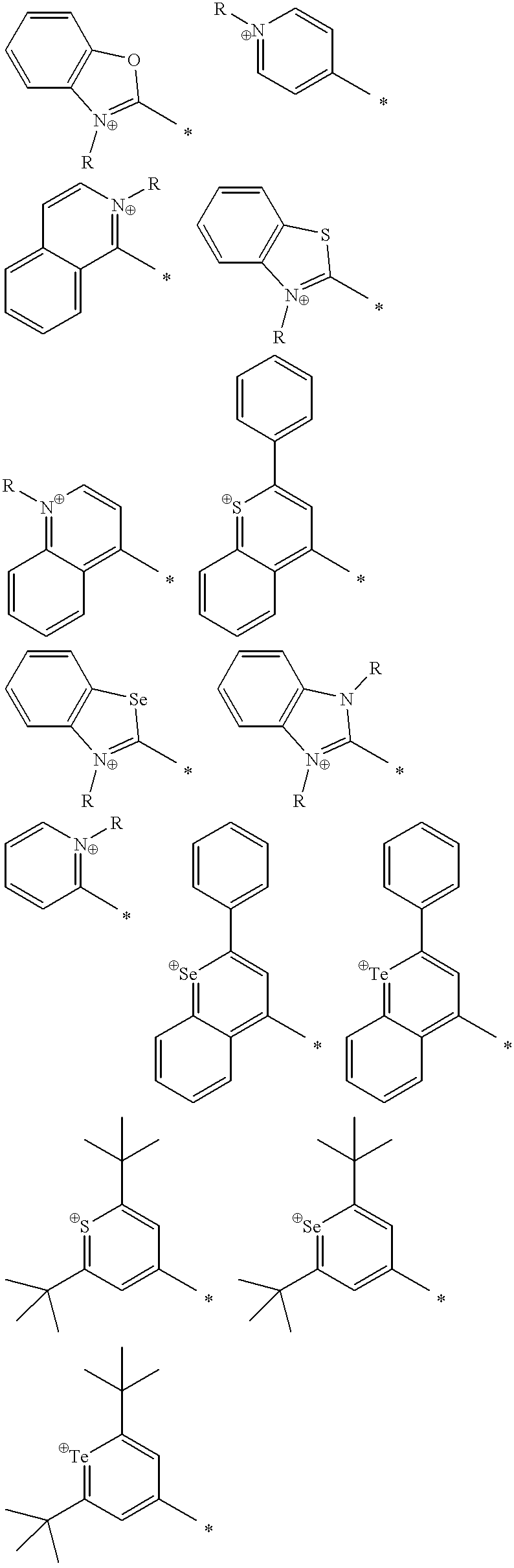
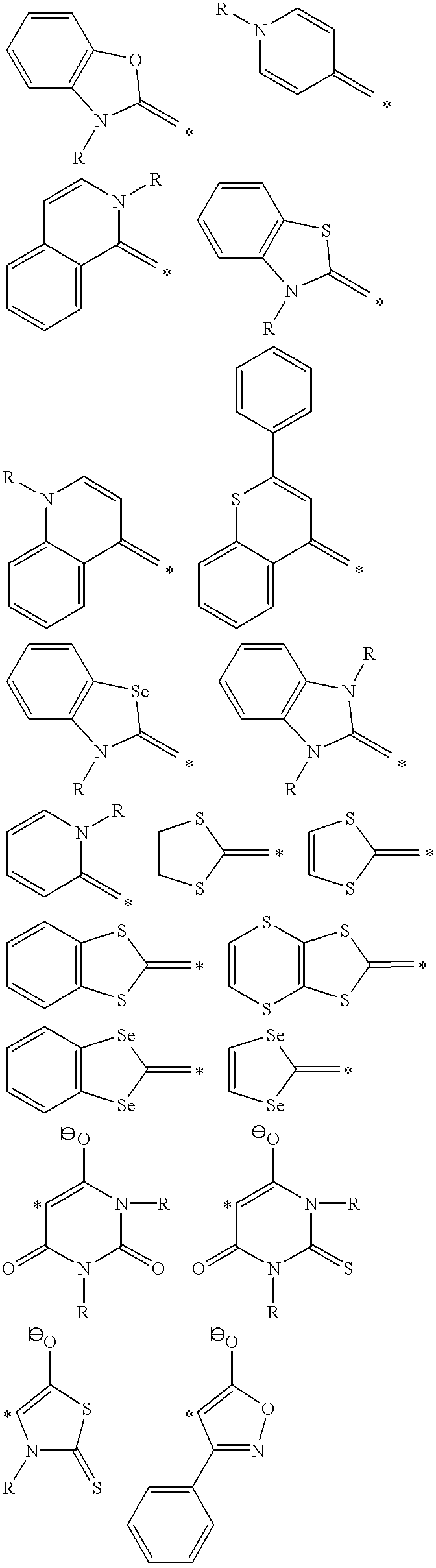
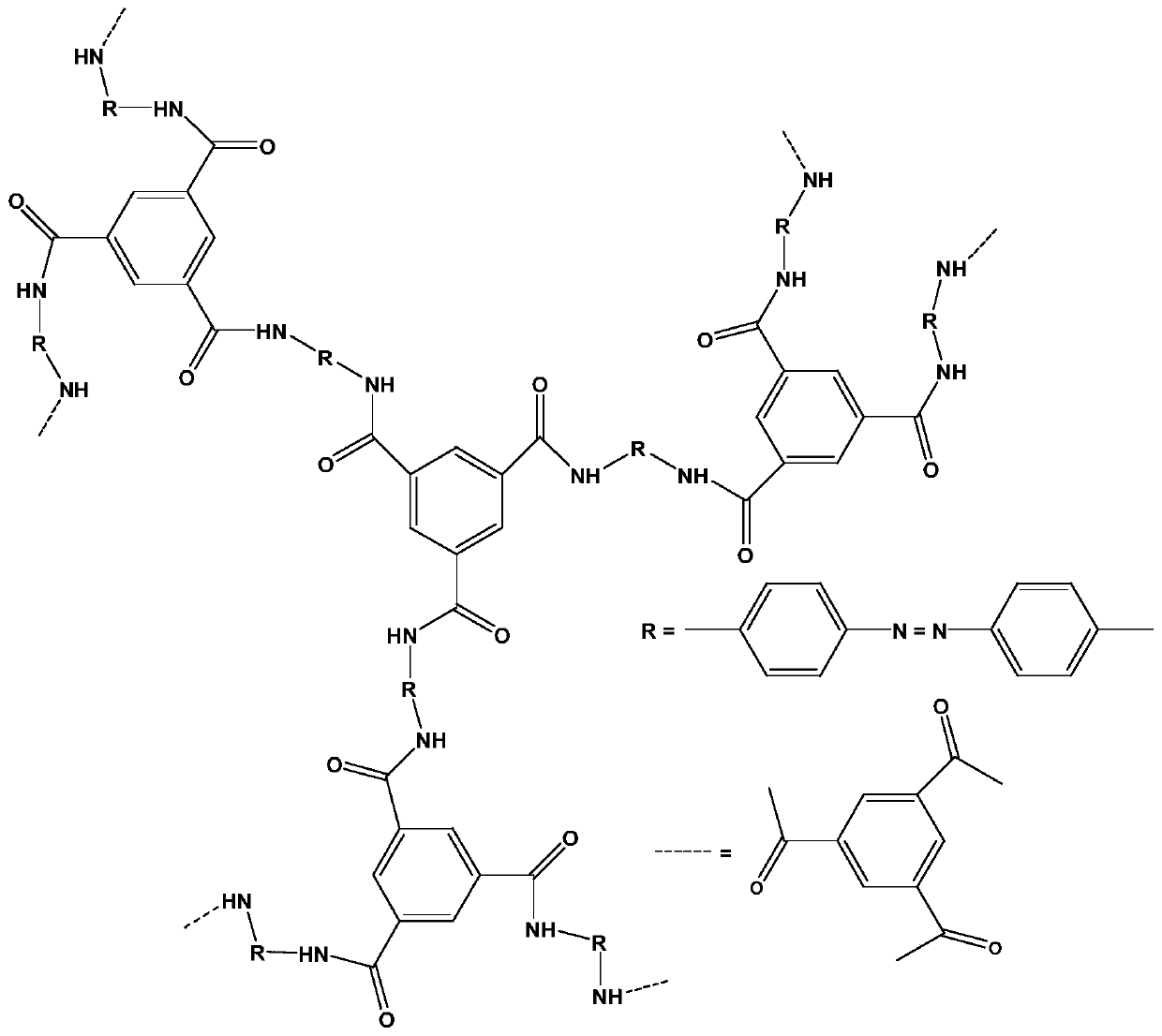
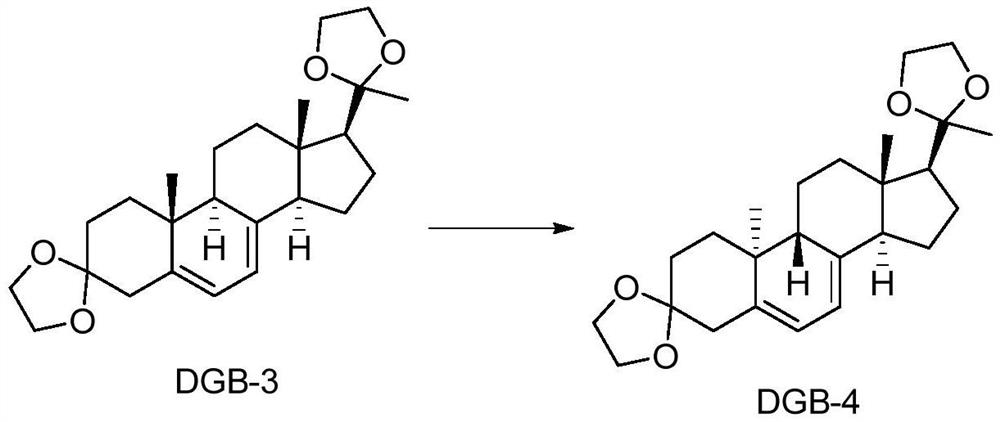
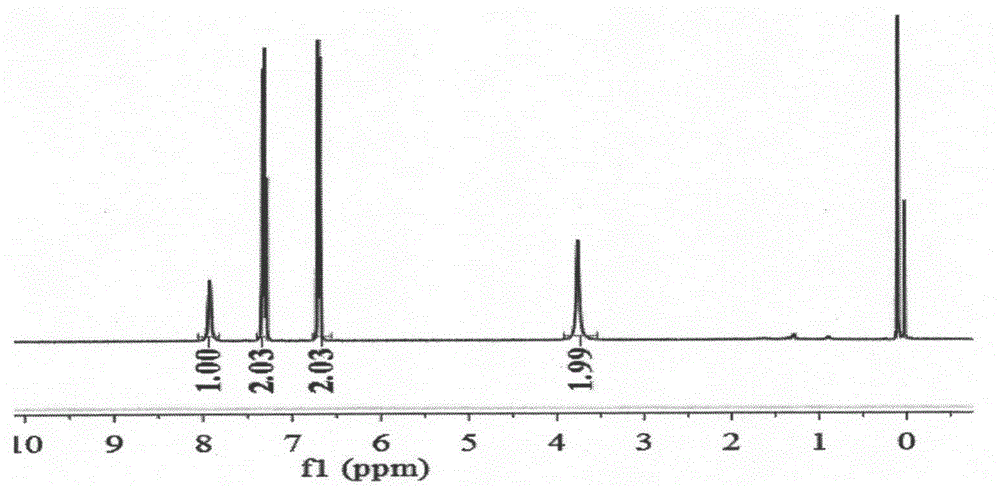
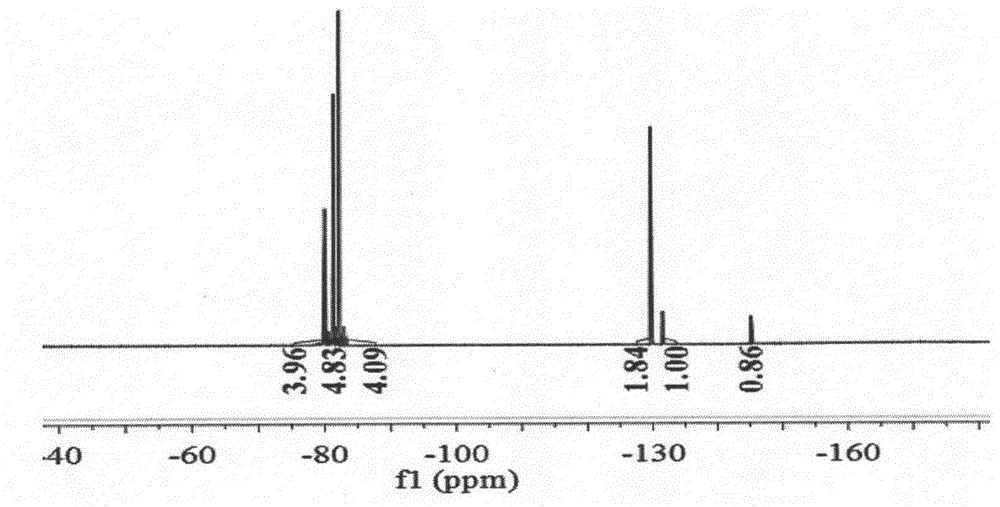
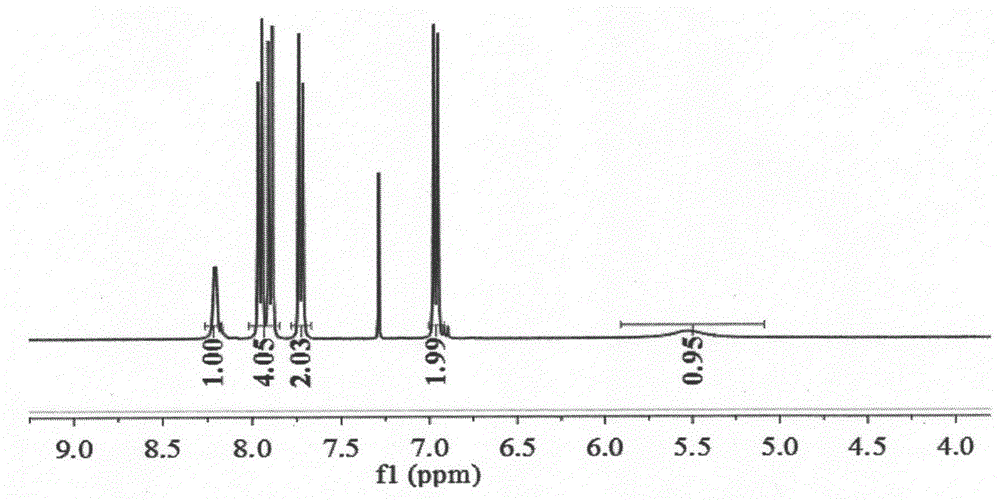
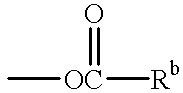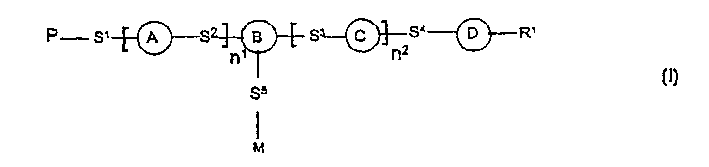Patents
Literature
119 results about "Photoisomerization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In chemistry, photoisomerization is a molecular behavior in which structural change between isomers is caused by photoexcitation. Both reversible and irreversible isomerization reactions exist. However, the word "photoisomerization" usually indicates a reversible process.
Process of changing the refractive index of a composite containing a polymer and a compound having large dipole moment and polarizability and applications thereof
InactiveUS6090332AReduce the valuePoor resolutionRecord information storageDigital storageThermoplasticBiological imaging
Fused ring bridge, ring locked dyes that form thermally stable photorfractive compositions. The fused ring bridge structures are pi -conjugated bonds in benzene-, naphthalene- or anthracene-derived fused ring systems that connect donor and acceptor groups. The donor and acceptor groups contribute to a high molecular dipole moment and linear polarizability anisotropy. The polarization characteristics of the dye molecules are stabilized since the bonds in the fused ring bridge are not susceptible to rotation, reducing the opportunity for photoisomerization. The dyes are compatible with polymeric compositions, including thermoplastics. The dyes are electrically neutral but have charge transport, electronic and orientational properties such that upon illumination of a composition containing the dye, the dye facilitates refractive index modulation and a photorefractive effect that can be utilized advantageously in numerous applications such as in optical quality devices and biological imaging.
Owner:CALIFORNIA INST OF TECH
Polymerised liquid crystal film with retardation or orientation pattern
ActiveUS7435357B2Liquid crystal compositionsPhotosensitive materialsPhotoisomerizationLiquid-crystal display
The invention relates to a polymerised liquid crystal (LC) film comprising at least one photoisomerisable compound and having a pattern of regions with different retardation and / or different orientation of the LC material, to methods of preparing such a film, and to its use as alignment layer, optical retardation film or optical waveguide in liquid crystal displays or other optical or electrooptical components or devices, or for decorative or security applications.
Owner:MERCK PATENT GMBH
Broad-band-cholesteric liquid-crystal film, process for producing the same, circularly polarizing plate, linearly polarizing element,illiminator, and liquid-crystal display
InactiveUS20060127605A1Reduce the number of manufacturing stepsReduce the numberLiquid crystal compositionsPolarising elementsPhotoisomerizationDisplay device
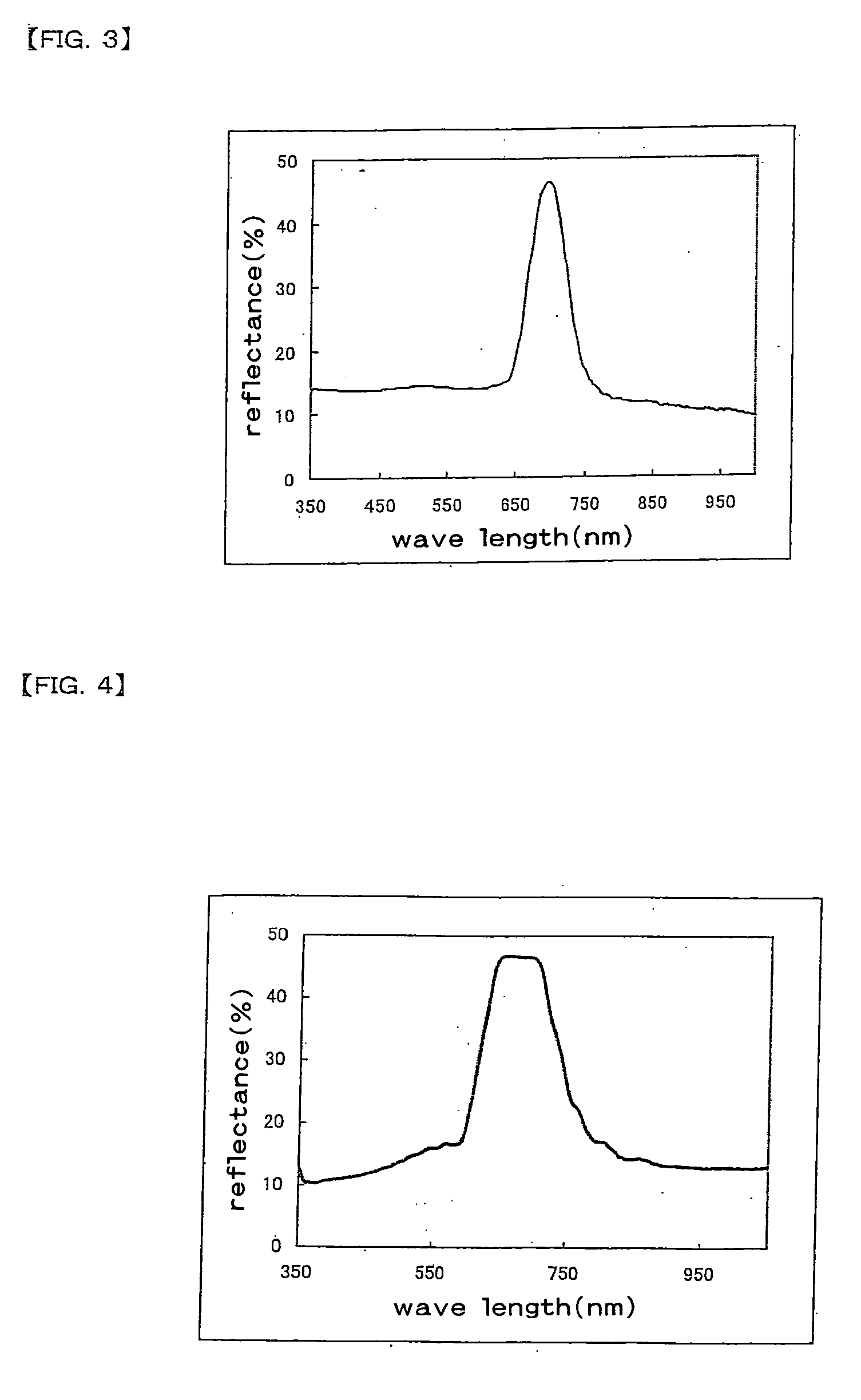
A broad band cholesteric liquid crystal film of the present invention is a cholesteric liquid crystal film obtained by coating a liquid crystal mixture containing a polymerizable mesogen compound (a), a polymerizable chiral agent (b) and a photoisomerizable material (c) on a substrate to ultraviolet polymerize a coat of the liquid crystal mixture, and has a reflection bandwidth of 200 nm or more. A broad band cholesteric liquid crystal film of the present invention has a broad reflection band, is of a thin type and can be manufactured in less of manufacturing steps.
Owner:NITTO DENKO CORP
Organic solar cell and method of fabricating the same
InactiveUS20090255586A1Reduce thicknessEnhanced light absorptionNanoinformaticsSolid-state devicesElastomerOrganic solar cell
An organic solar cell and a method of fabricating the same are provided. The organic solar cell includes a first electrode and a second electrode. An organic active layer is disposed between the first electrode and the second electrode. The organic active layer includes an concave-convex pattern in a surface adjacent to the second electrode. The concave-convex pattern may be formed by contacting an elastomer stamp and a top surface of the organic active layer. The elastomer stamp may be formed by molding using a template having a surface relief grating (SRG). The template may include a photoisomerization polymer layer, and the surface relief grating may be formed by irradiating interference light onto the photoisomerization polymer layer. The surface relief grating may be a blazed diffraction grating.
Owner:GWANGJU INST OF SCI & TECH
Antibacterial composition for topical administration containing antibiotic
The present invention provides a safe antibacterial composition for topical administration which stably contains a penem antibiotic having a broad-spectrum and potent antibacterial activity while otherwise being chemically susceptible to hydrolysis, oxidation, photoisomerization or the like. The compositions of the present invention comprise an antibacterial composition for topical administration comprising a penem antibiotic or a pharmaceutically acceptable salt thereof in a non-aqueous base.
Owner:DAIICHI SUNTORY PHARMA CO LTD
Triazine ring based polymers for photoinduced liquid crystal alignment, liquid crystal alignment layer containing the same, liquid crystal element using the alignment layer and method of manufacturing the same
InactiveUS20040039150A1Easy alignmentImprove stabilityLiquid crystal compositionsOrganic chemistryCrystallographyPhotoisomerization
Triazine ring based polymers for photoinduced liquid crystal alignment introduces photoactive groups for inducing, reinforcing, improving and preserving liquid crystal alignment, for example photoreactor such as cinnamate, coumarin, chalcone and maleimide, as a chain to have at least one photoactive group. One of the photoactive groups can experience Fries rearrangement which induces liquid crystal alignment, and other groups can experience photodimerization, photoisomerization, photocrosslinking or photodegradation to reinforce, change or preserve the generated alignment.
Owner:LS MTRON LTD
Electrochemical and photochemical electrodes and their use
InactiveUS6350368B1Increase and decreases electrically induced catalytic activityEfficient and rapid electron transferMicrobiological testing/measurementPreparing sample for investigationElectricityPhotoisomerization
Electrodes carrying FAD-dependent enzymes on their surface are enclosed. The FAD is modified to include a functional group or moiety which affects the properties of the enzyme. The functional group or moiety can be a binding moiety through which the FAD-enzyme complex is immobilized onto the electrode; it can be an electron mediator group for transferring electrons between the electrode and the FAD; or it can be a photoisomerizable group which can undergo photoisomerization which yields a change in the rate of the electrically induced catalytic activity of the enzyme. The electrodes can be used in electrochemical systems for deforming analytes in liquid medium or for recordal of optical signals.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
Cyano group-substituted stilbene-type liquid crystal material and its preparation technology and use
InactiveCN103710030ARealize dynamic adjustmentHas photoisomerization propertiesLiquid crystal compositionsCarboxylic acid nitrile preparationPhotoisomerizationBenzene
The invention discloses a cyano group-substituted stilbene-type liquid crystal material and its preparation technology and use. A liquid crystal fluorescent display utilizes the novel cyano group-substituted stilbene-type liquid crystal material. The cyano group-substituted stilbene-type liquid crystal material is a good liquid crystal material, has fluorescence radiation properties and can produce photoisomerization under ultraviolet irradiation. Fluorescent molecular dispersed liquid crystals are prepared by doping of the cyano group-substituted stilbene-type liquid crystal material and nematic liquid crystals, and the mixture is poured into a liquid crystal box. The mixture in the liquid crystal box produces photoisomerization under ultraviolet irradiation so that the cyano group-substituted stilbene-type liquid crystal material and the nematic liquid crystals produce phase separation. An AC voltage is applied to two ends of the liquid crystal box, and through power supply opening and closing, the dynamically reversible adjustable electrically-controlled liquid crystal fluorescent display is prepared. Based on the above properties, the electrically-controlled liquid crystal fluorescent display has a wide application prospect in the modern displaying and photoelectric field.
Owner:HEFEI UNIV OF TECH
Azobenzene polymer material and preparation method and applications thereof
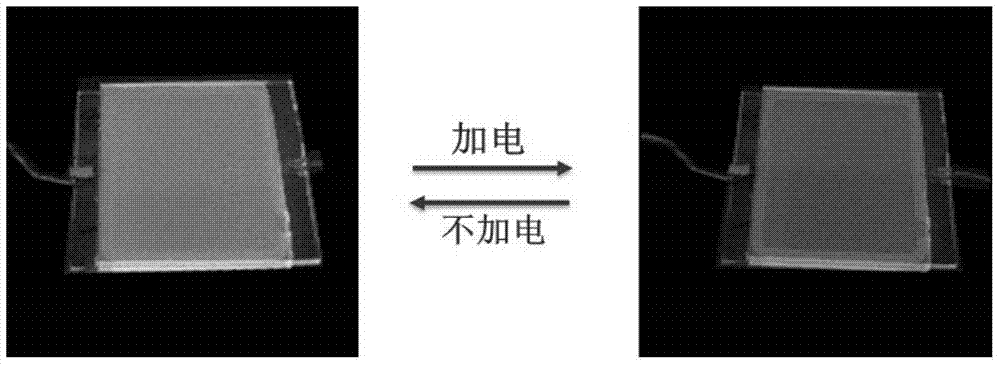
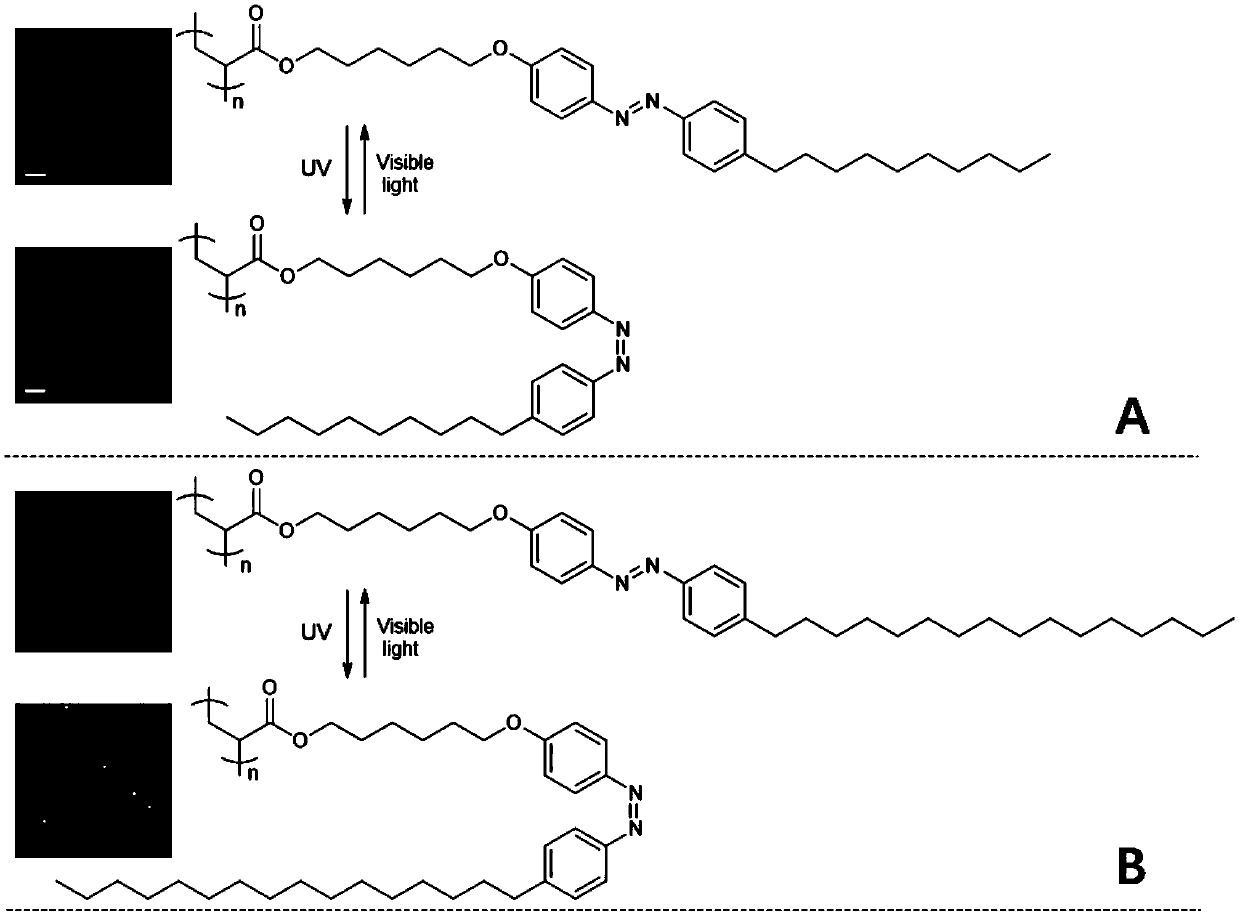
ActiveCN109651545AHas photoremediation propertiesAdhesiveEster polymer adhesivesPhotoisomerizationPolymer science
The invention provides an azobenzene polymer material which has a structure represented by formula I shown in the description. The azobenzene polymer can be isomerized under photoinduction, can be changed in glass-transition temperature, can produce a reversible solid-liquid transition at room temperature, and has and has repeatable self-repairing and surface repairing effects. Meanwhile, a trans-azobenzene polymer is solid, has strong adhesion and can bear loads. In contrast, a cis-azobenzene polymer is liquid and has weak adhesion, which means photoisomerization can cause load drop. Therefore, the azobenzene polymer provided by the invention can be used as a photoresponsive self-repairing material and a light-controlled reversible adhesive, and can solve problems a conventional thermoplastic polymer material in plastics, remodeling and damage repair of a coating, separation, recycling and adhesive surface repair of an adhesive and the like.
Owner:UNIV OF SCI & TECH OF CHINA
Liquid crystal display element and method for manufacturing same
ActiveUS20150275089A1Improved image sticking characteristicGood in alignment stabilityLiquid crystal compositionsCoatingsPolyamideEngineering
The present invention relates to a liquid crystal display device including a pair of a substrate arranged opposite to each other, an electrode group disposed on one side or both sides of the pair of substrates facing each other, a plurality of active devices connected to the electrode group, a liquid crystal alignment film disposed on each facing side of the pair of substrates, and a liquid crystal layer interposed between the pair of substrates, wherein the liquid crystal alignment film is manufactured by irradiating linearly polarized light to a film obtained from polyamic acid or a derivative thereof having a photoisomerization structure in the main chain or a film being subject to heat-baking so as to provide alignment-controlling capability, and the liquid crystal layer is a liquid crystal composition having negative (−) dielectric anisotropy. By the present invention, a liquid crystal display device having improved image sticking characteristics and good alignment stability is provided.
Owner:JNC CORP +1
Method for preparing light-controlled solid-liquid transformed azo polymer
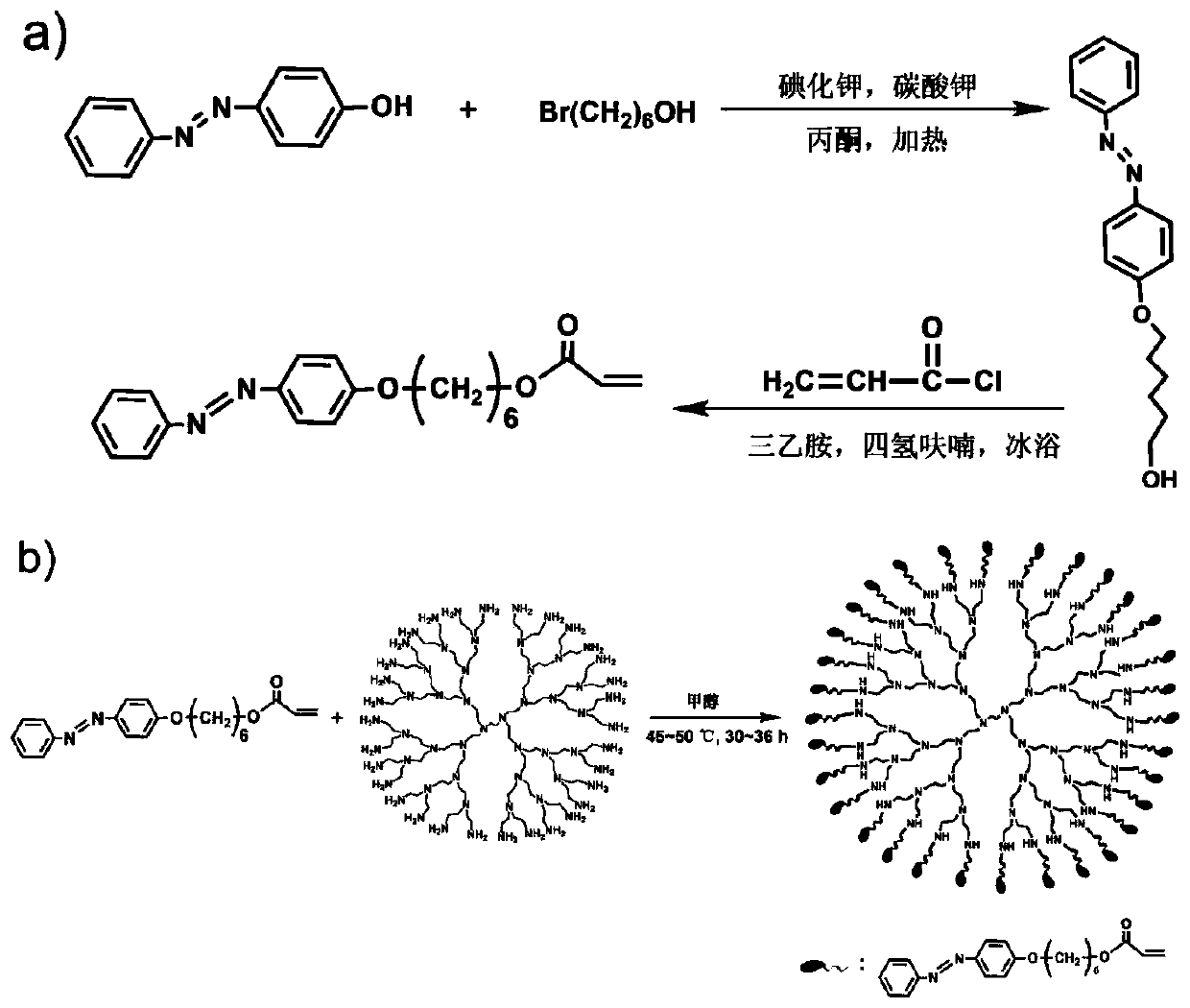
ActiveCN110437383AHigh molecular weightGood film formingHeat-exchange elementsDendrimerUltraviolet lights
The invention relates to a method for preparing a light-controlled solid-liquid transformed azo polymer, and belongs to the field of polymer functional materials. The azo polymer is prepared by azobenzene acrylate and polyamidoamine (PAMAM) dendrimer through michael addition reaction. The azobenzene in the azo polymer has reversible trans-cis photoisomerization properties. The azo polymer can realize reversible solid-liquid phase transformation under light control, specifically, azobenzene changes from a trans structure to a cis structure under ultraviolet light irradiation, and the azo polymer changes from a solid state to a liquid state; and the azobenzene under visible light irradiation transforms from the cis structure to the trans structure, and the azo polymer changes from a liquid state to a solid state. The method provides a new strategy for developing novel light-induced solid-liquid transformed materials, light-induced phase change heat storage and molecular photothermal storage materials.
Owner:UNIV OF SCI & TECH BEIJING
Photoisomerization reflecting mirror system
ActiveCN103472567AReduce development costsReduce weightMirrorsMountingsPhotoisomerizationComposite film
Disclosed is a photoisomerization reflecting mirror system. Azobenzene derivative composite films having photoisomerization features are adopted as materials of a reflecting mirror, and the surface shape of the film mirror is adjusted through the mode that the irradiation direction, time and power of polarized light to the film mirror are controlled to satisfy imaging precision requirements of an optical system. The photoisomerization reflecting mirror system mainly comprises a film reflecting mirror assembly, a reflecting mirror assembly supporting structure, a scanning device, a polarization and energy control device, a beam expansion device and a laser device; laser emitted by the laser device can be converted to light beams satisfying system control requirements through the beam expansion device and the polarization and energy control device; the scanning device adjusts the directions of the light beams, irradiates appointed regions and adjusts the surface shape of the reflecting mirror through the photoisomerization features of the materials. The photoisomerization reflecting mirror system has the advantages of being light and controllable in surface shape precision, and can be made into a large-diameter reflecting mirror, be applied to space remote sensors, reduce weights of the remote sensors and save the development cost of the remote sensors.
Owner:BEIJING RES INST OF SPATIAL MECHANICAL & ELECTRICAL TECH
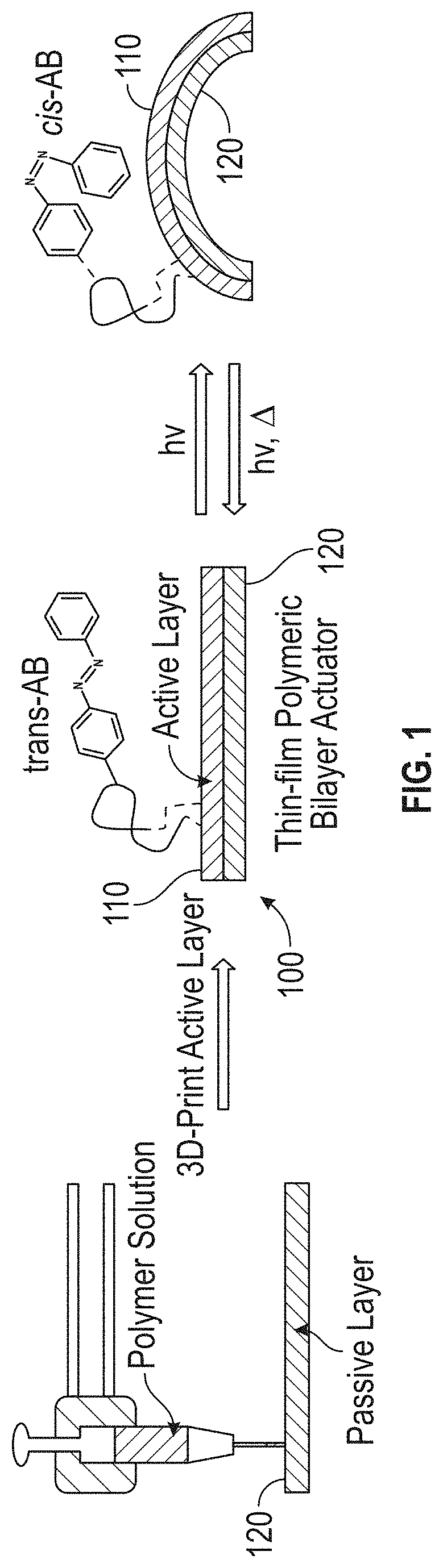
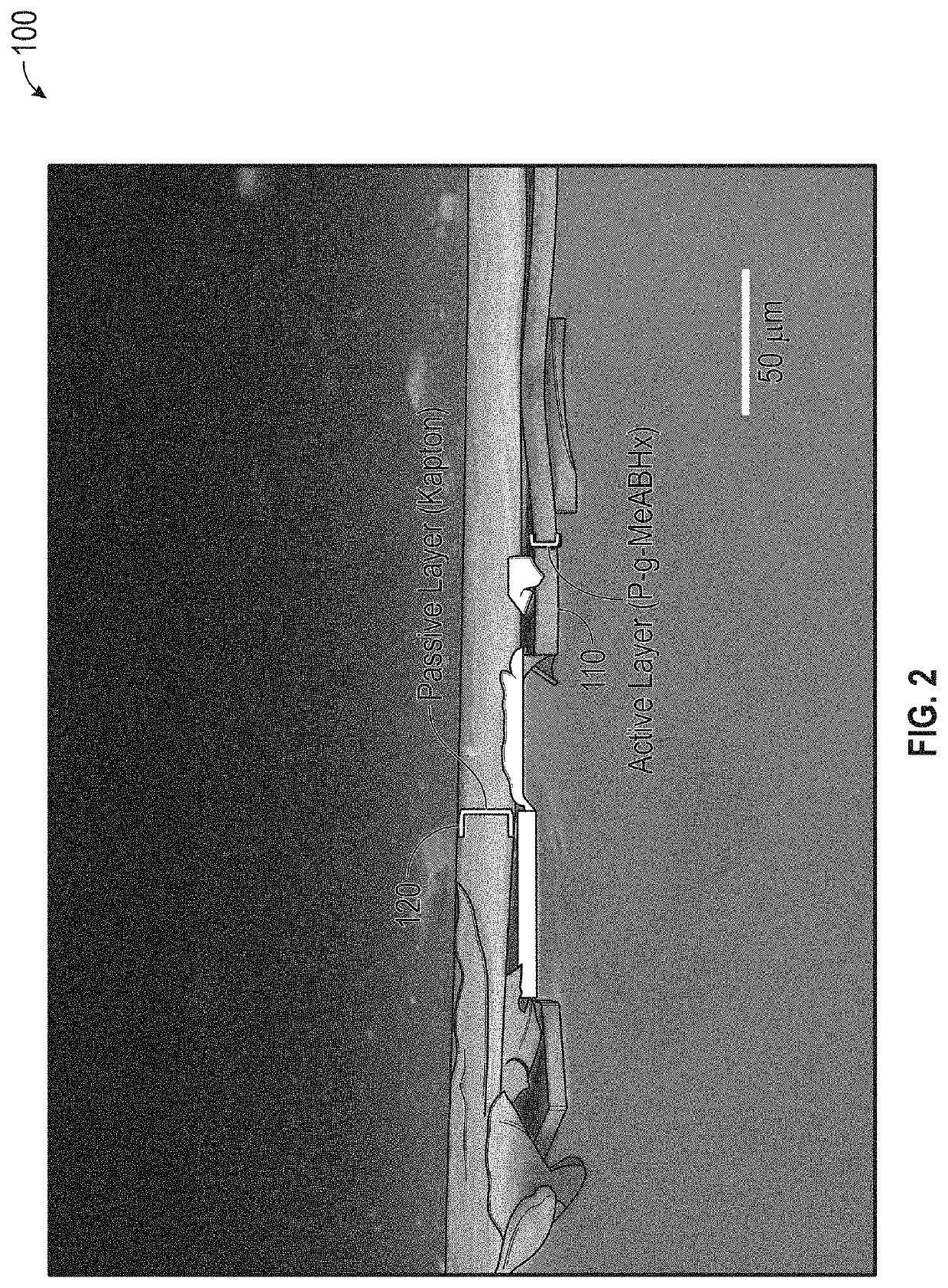
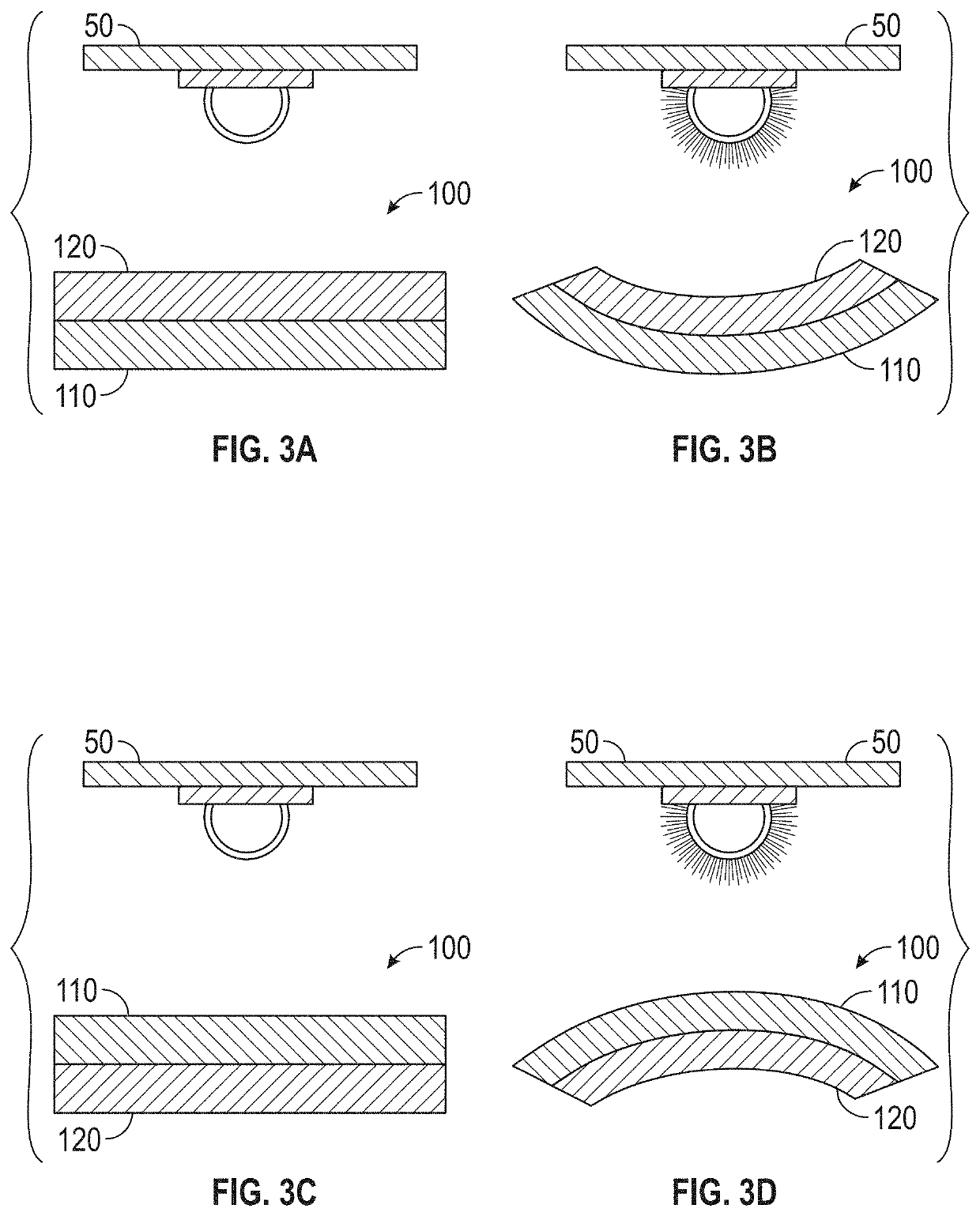
Externally Activated Shape Changing Device
ActiveUS20190367692A1Reduce the amount requiredReduce the number of partsAdditive manufacturing apparatusMachines/enginesShape changeEngineering
The present invention provides a 4D printed component that uses the photoisomerization stimulus as a method of activation. Other 4D printing methods use heat, moisture, a combination of heat and stress, and the heat from a light source as methods of activation. The present invention takes advantage of 3D printing capability and adds the capability of providing a printable material that dynamically changes shape over time when exposed to an external stimulus. The invention reduces the number of required 3D printed parts to create a moving object. This characteristic reduces the amount of onboard weight of the 3D printed components by reducing the number of parts required to create motion. The present invention removes the need for onboard sensors, processors, motors, power storage, etc. This characteristic will allow for manufacturing of, inter alia, novel medical devices, automated actuators, packaging, smart textiles, etc.
Owner:DREXEL UNIV
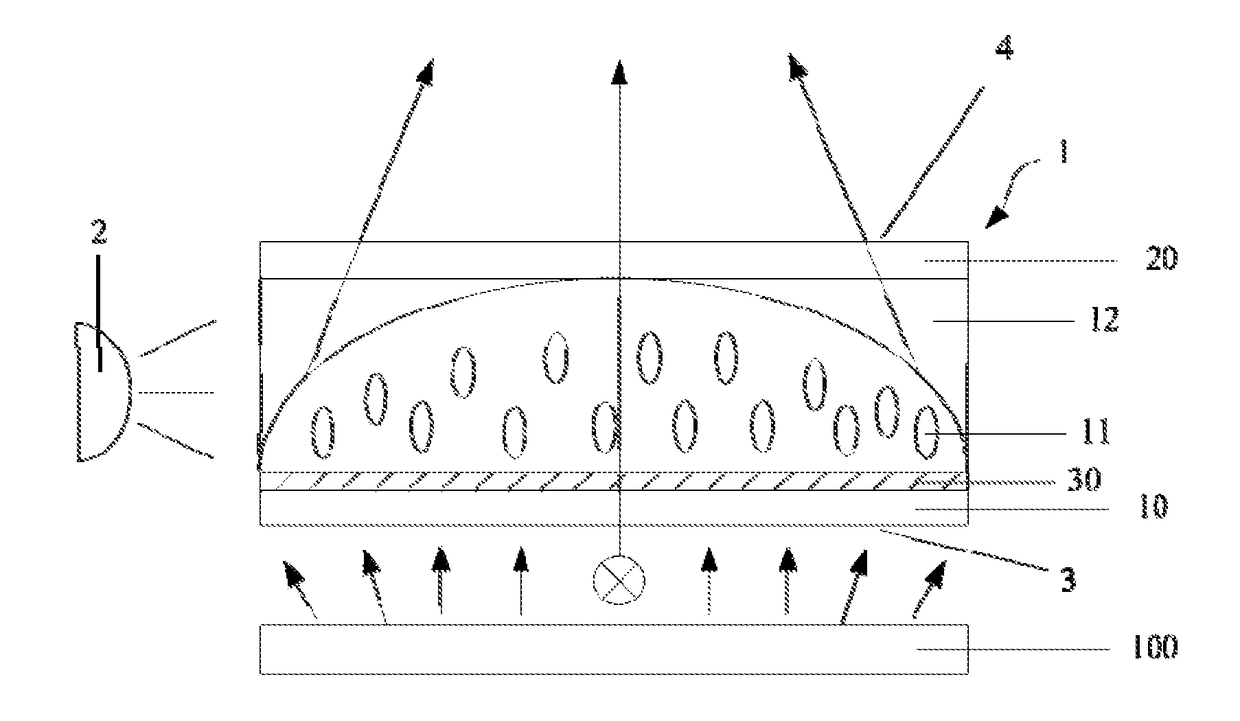
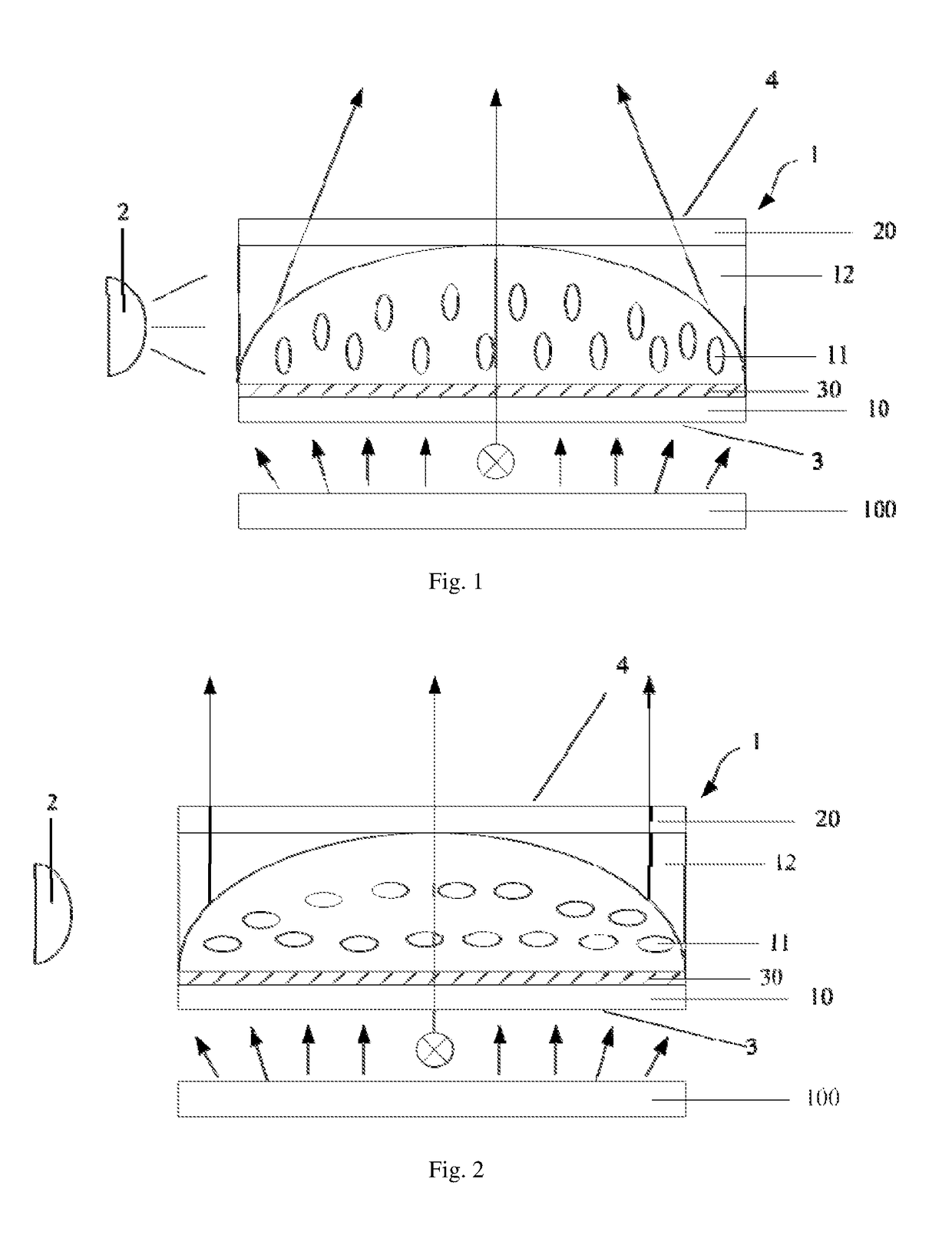
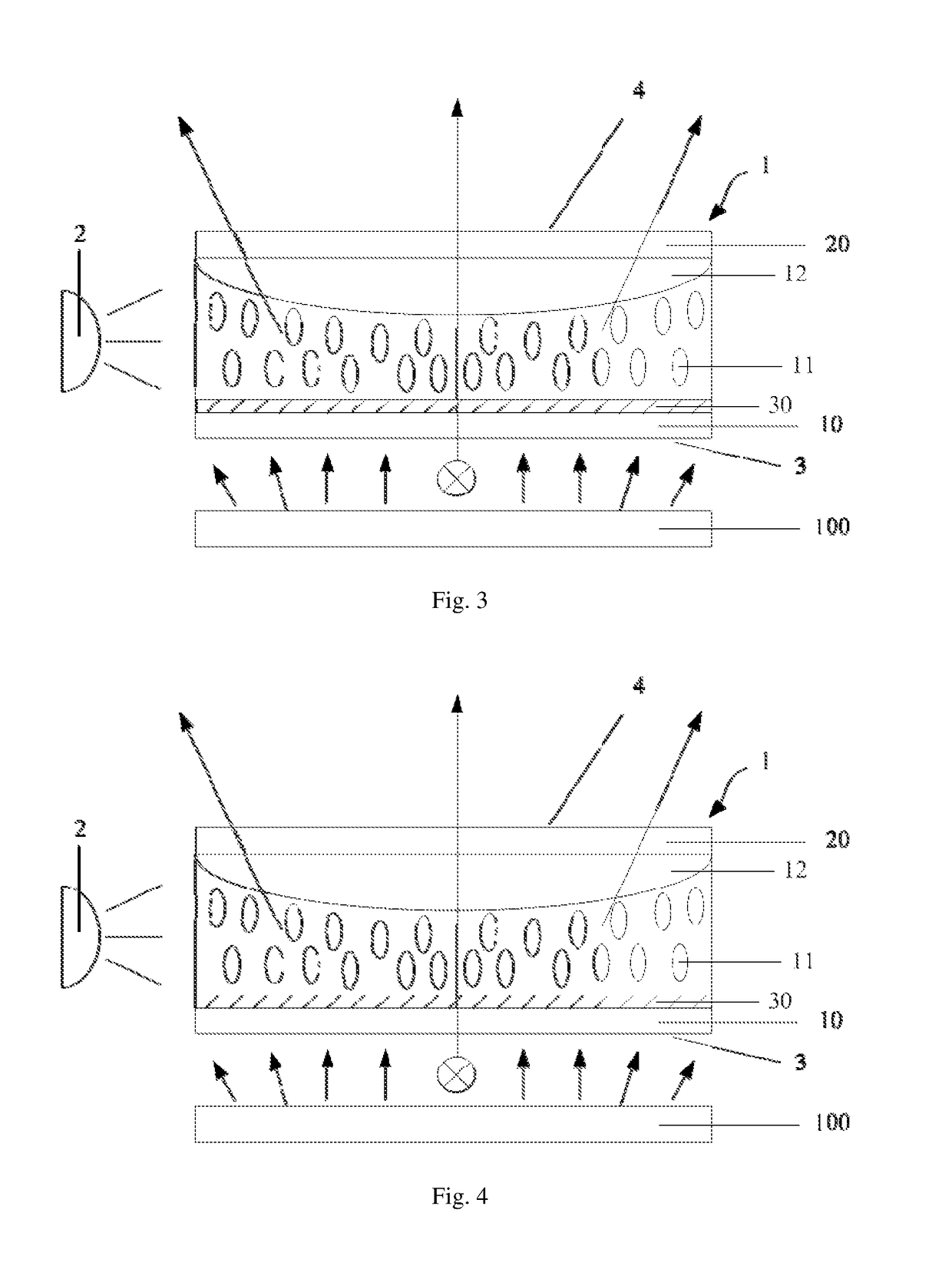
Optical device, display apparatus and driving method thereof
An optical device for a display panel. The optical device comprises an optical element (1). The optical element (1) may include a first surface (3) and a second surface (4) opposite the first surface, and side surfaces. The optical device may further comprise a light source (2) facing one of the side surfaces of the optical element (1). The optical element (1) may comprise a compound containing a photoisomer group. The photoisomer group may undergo a photoisomerization under an irradiation of the light source. Accordingly, an illumination area by a light passing through the first surface (3) and exiting from the second surface (4) may increase or decrease.
Owner:BOE TECH GRP CO LTD
Liquid crystal orientation liquid for light orientation processing technique, and liquid crystal orientation film employing same
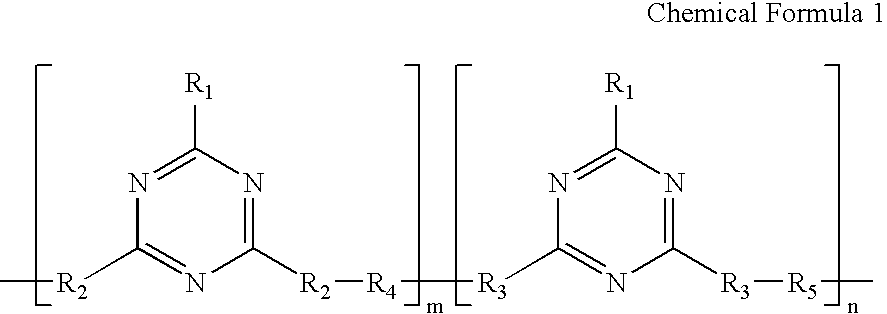
Provided are a liquid crystal orientation film with which it is possible to alleviate an afterimage resulting from AC driving which occurs with an IPS drive format or an FFS drive format liquid-crystal display element, and a liquid crystal orientation liquid for a light orientation processing technique for said film. A liquid crystal orientation liquid for a light orientation processing technique includes a constituent (A), a constituent (B), and an organic solvent, wherein the degree of inclusion of the constituent (B) is 0.1-15 parts to 100 parts of the constituent (A). Constituent (A): one or more polymers, wherein by illuminating with polarized light, one of the following reactions progresses: photolysis, photodimerization, or photoisomerization, and wherein an anisotropy is applied in either the same direction as the polarized light direction or the perpendicular direction to the polarized light direction. Constituent (B): a polymer having a structure which is represented with formula (1), following: (Chemical Formula 1) (Wherein W1 and W2 are independent bivalent organic groups including an aromatic group with 6-30 carbons, and A is a bivalent organic group including an alkylene group with 2-20 carbons.
Owner:NISSAN CHEM IND LTD
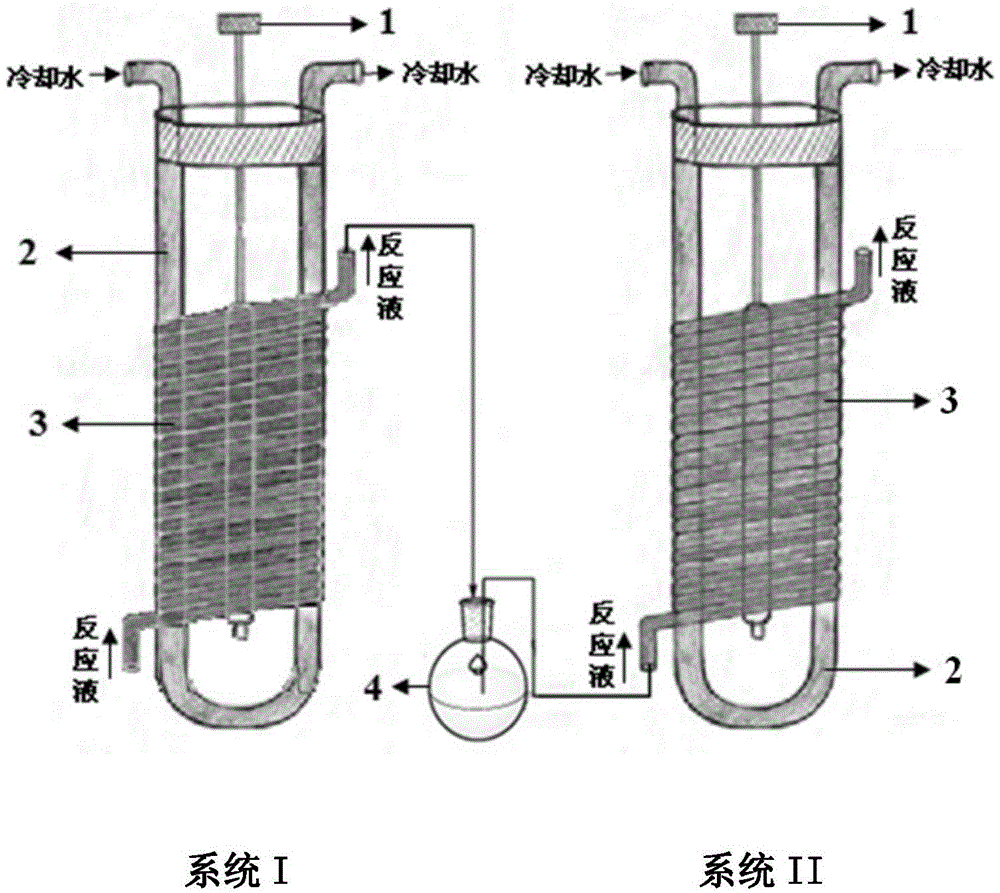
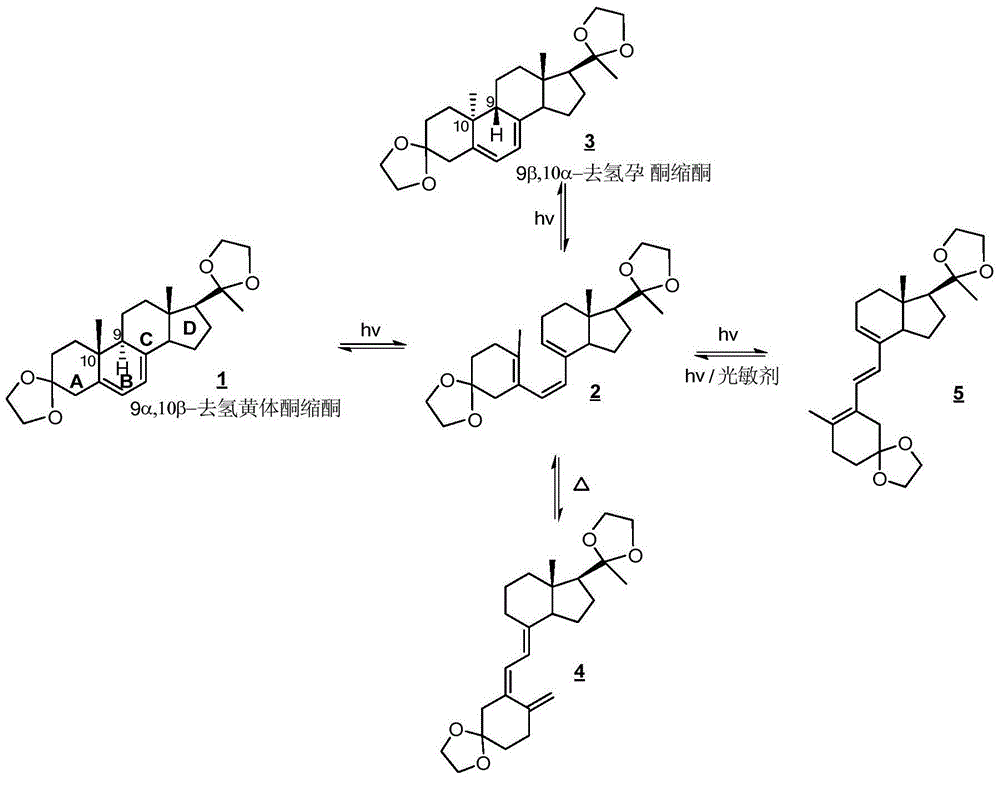
Method for preparing 9β, 10-α-dehydroprogesterone ketal by dual-wavelength microfluidic technology and dual-wavelength microfluidic photochemical reactor
ActiveCN103848880BHigh yieldEasy to operateSteroidsEnergy based chemical/physical/physico-chemical processesPhotoisomerizationPeristaltic pump
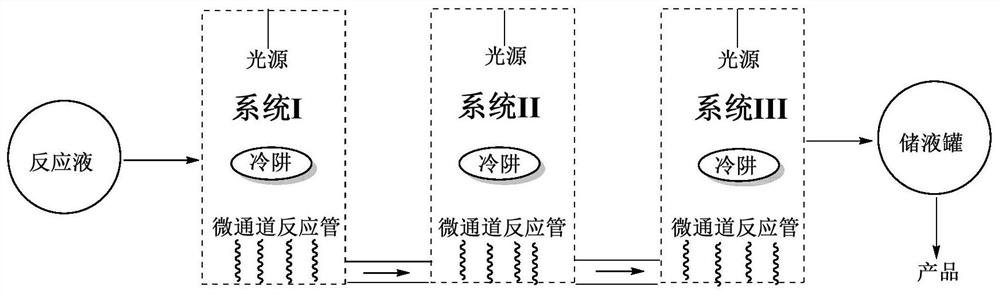
The invention relates to a method for preparing 9beta,10-alphat-dehydroprogesterone ketal by using a dual-wavelength microflow technology and a dual-wavelength microflow photochemistry reactor. The method comprises the following steps: performing illumination reaction in the dual-wavelength microflow photochemistry reactor under the nitrogen protection, regulating and controlling flow velocity of photochemistry reaction liquor in a system I and a system II forming the dual-wavelength microflow photochemistry reactor by using a peristaltic pump. The conversion rate of the 9beta,10-alphat-dehydroprogesterone ketal after the illumination reaction of the system I achieves 87-92%, after the illumination reaction of the system I, in terms of the consumption of the 9beta,10-alphat-dehydroprogesterone ketal, the yield of the 9beta,10-alphat-dehydroprogesterone ketal is up to 46.3%. Compared with the traditional kettle-type photochemistry reaction, the amount of the byproduct in the photoisomerization reaction is reduced to less than 2.1% through the dual-wavelength microflow photochemistry reaction technology, and the yield of the target product 9beta,10-alphat-dehydroprogesterone ketal is greatly improved.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Film forming material and preparation of surface relief and optically anisotropic structures by irradiating a film of the said material
InactiveUS8026021B2Improve induction efficiencySimple materialRadiation applicationsMouldsGratingMixed materials
The present invention is directed to a film forming, photoactive, homogeneously mixed material comprising a complex prepared from (a) at least one ionic photosensitive compound which may undergo a photoreaction, selected from photoisomerizations, photocycloadditions and photoinduced rearrangements, and / or (a′) at least one photosensitive polyelectrolyte (“second polyelectrolyte”) carrying residues which may undergo said photoreaction, and (b) at least one (“first”) polyelectrolyte carrying charges which are opposite to those of the active groups of the photosensitive material. This material has unique photochemical properties in that non-scattering, optically clear films may prepared therefrom which allow light-induced generation of optical anisotropy and of topological surface structures, e.g. such as surface relief gratings (SRG).
Owner:FRAUNHOFER GESELLSCHAFT ZUR FOERDERUNG DER ANGEWANDTEN FORSCHUNG EV
Thermally stable molecules with large dipole moments and polarizabilities and applications thereof
Disclosed are fused ring bridge, ring-locked dyes that form thermally stable photorefractive compositions. The fused ring bridge structures are pi-conjugated bonds in benzene-, naphthalene- or anthracene-derived fused ring systems that connect donor and acceptor groups. The donor and acceptor groups contribute to a high molecular dipole moment and linear polarizability anisotropy. The polarization characteristics of the dye molecules are stabilized since the bonds in the fused ring bridge are not susceptible to rotation, reducing the opportunity for photoisomerization. The dyes are compatible with polymeric compositions, including thermoplastics. The dyes are electrically neutral but have charge transport, electronic and orientational properties such that upon illumination of a composition containing the dye, the dye facilitates refractive index modulation and a photorefractive effect that can be utilized advantageously in numerous applications such as in optical quality devices and biological imaging.
Owner:CALIFORNIA INST OF TECH
Photochromic hyperbranched azopolyamide and preparation method thereof
InactiveCN110343242APhotoresponsiveSpecial Photoelectric PropertiesTenebresent compositionsPhotoisomerizationAzo polymer
The invention provides a photochromic hyperbranched azopolyamide and a preparation method thereof. The method includes: taking 4, 4'-azodianiline and trimesic acid as the raw materials, firstly converting trimesic acid into trimesoyl chloride, and then utilizing the reaction activity of acyl chloride group and amino to synthesize hyperbranched azopolyamide by low temperature solution polycondensation. The photochromic hyperbranched azopolyamide has the optical activity of azo group, and also has good thermodynamic stability and excellent processability of polyamide substances, and a UV-Vis spectrum is employed to study the photoisomerization characteristics and photo-trans-cis isomerization kinetics of the synthesized azo polymer.
Owner:LINGNAN NORMAL UNIV
Method for manufacturing liquid crystal display device
InactiveUS20160178969A1Improve display qualityAvoid failureTube/lamp screens manufactureNon-linear opticsPhotoisomerizationIn plane
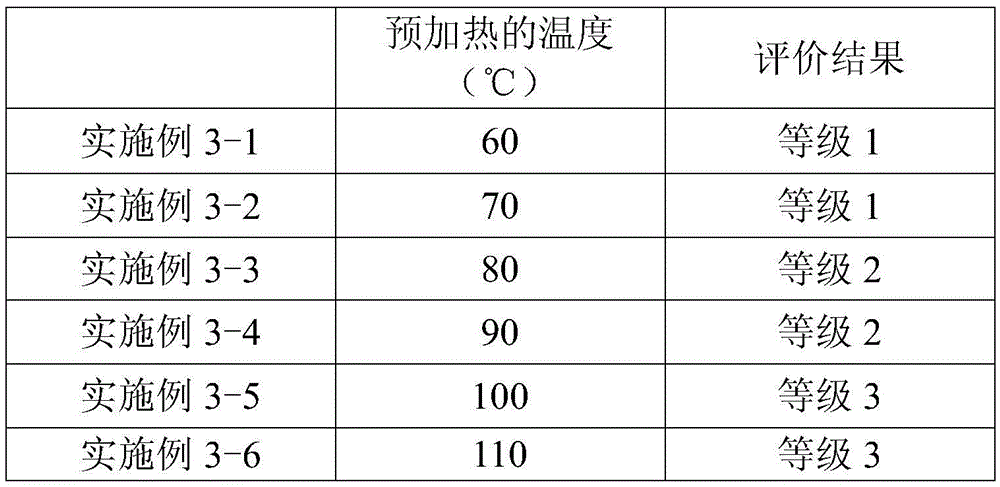
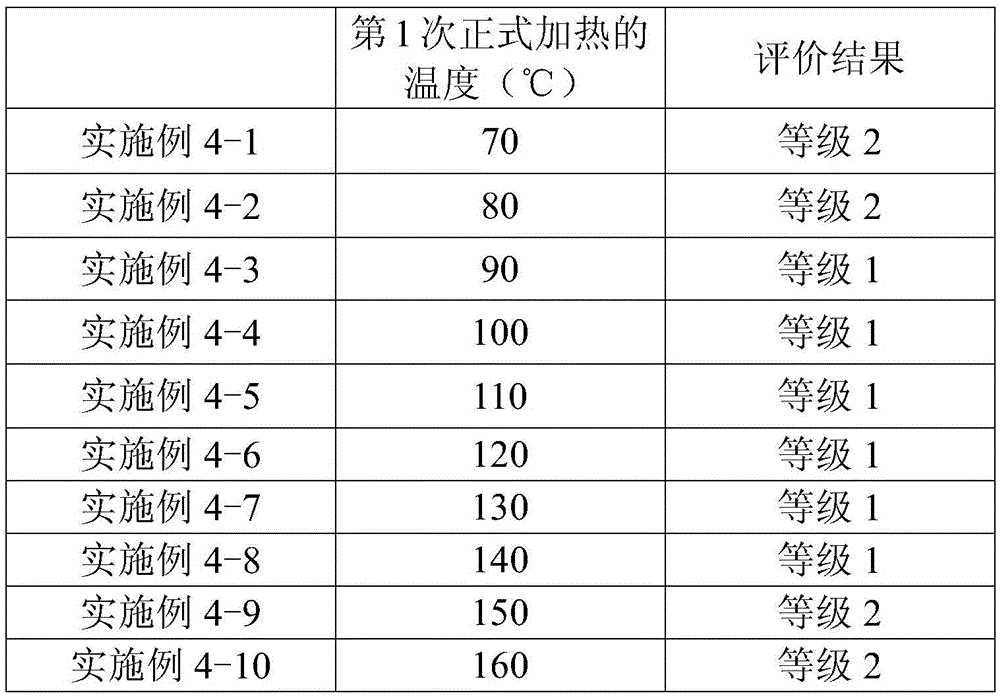
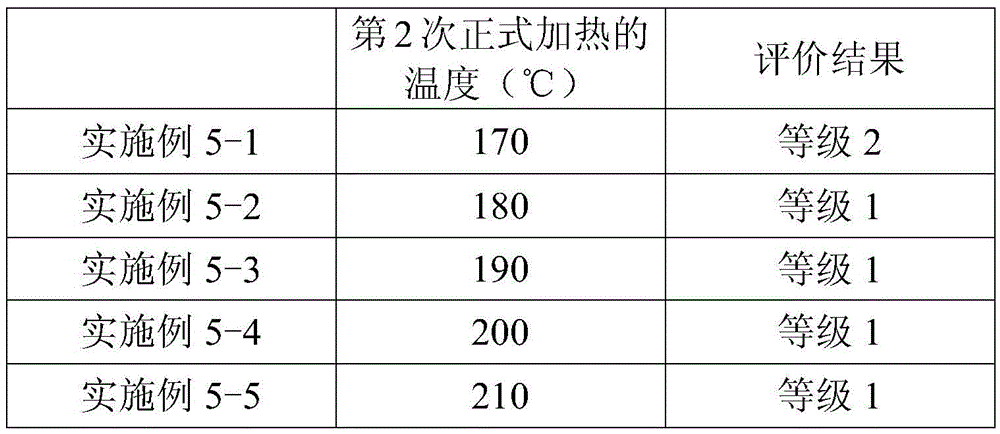
The present invention provides a method for manufacturing a liquid crystal display device including a photo-alignment film and capable of sufficiently improving the display quality. The method for manufacturing a liquid crystal display device of the present invention is a method for manufacturing a liquid crystal display device including a photo-alignment film. The method for manufacturing a liquid crystal display device successively includes a step (1) of forming on a substrate a film from a photo-alignment-film material that contains a solvent and a polymer including a photo-functional group that is capable of causing at least one chemical reaction selected from the group consisting of photodimerization, photoisomerization, and photo-Fries rearrangement; a step (2) of pre-heating the film to evaporate the solvent; a step (3) of irradiating the pre-heated film with polarized light; and a step (4) including an operation of main-heating the polarized-light-irradiated film at multiple temperatures from a lower temperature to a higher temperature. The liquid crystal display device is of an in-plane switching mode or a fringe field switching mode in each of which a pre-tilt angle is substantially 0°.
Owner:SHARP KK
Light-activated actuator element
InactiveUS20100016608A1Rapid responseSmall sizeOrganic chemistryMachines/enginesUltraviolet lightsLight driven
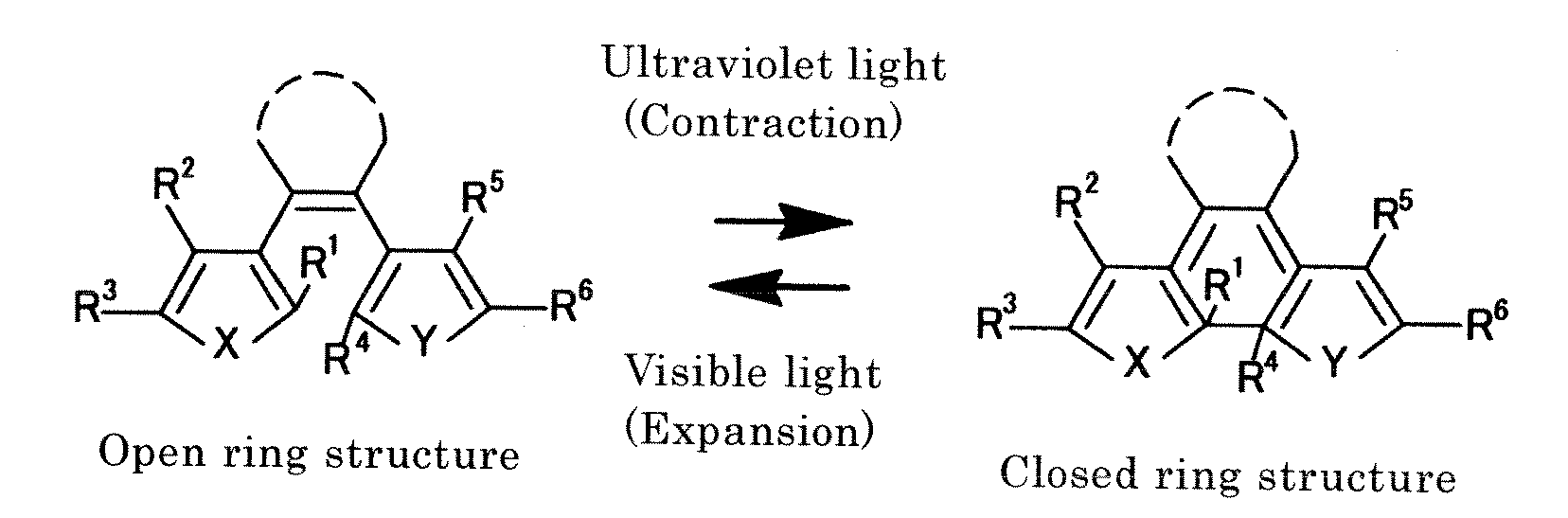
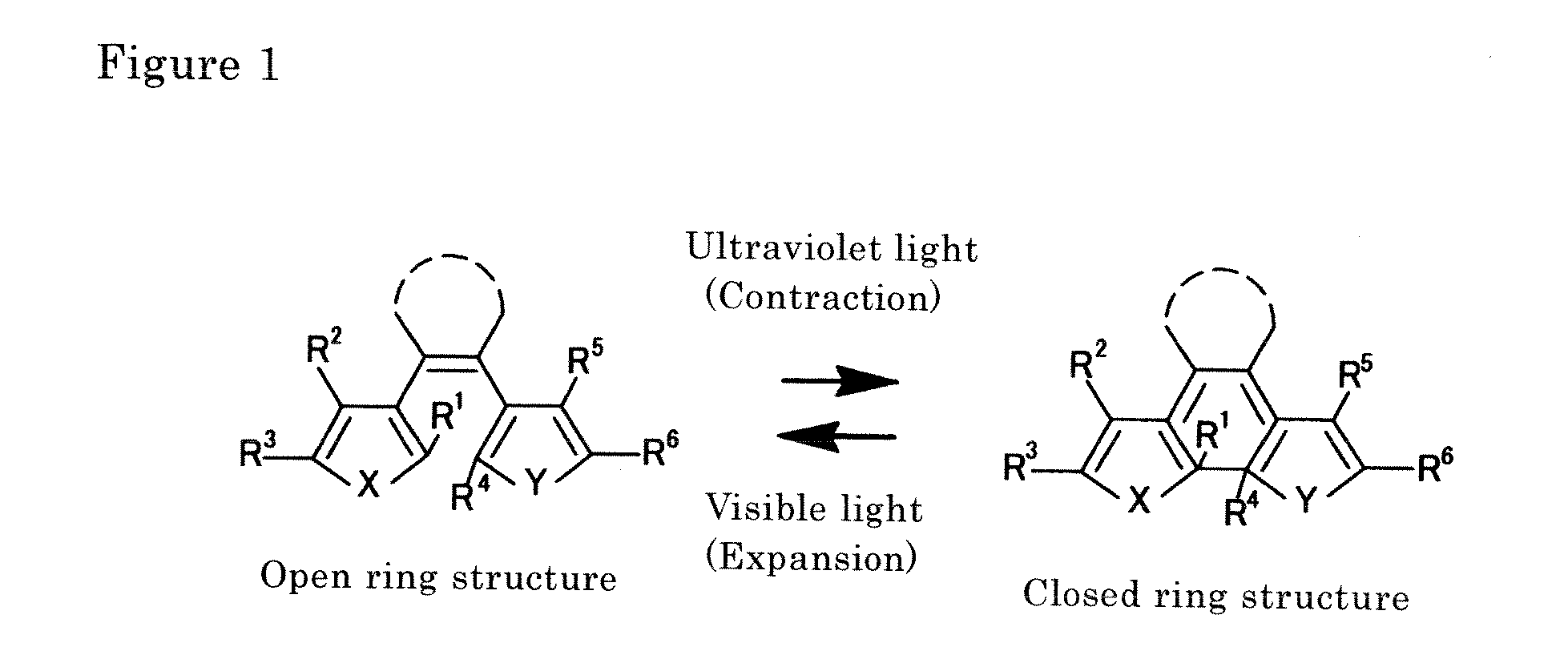
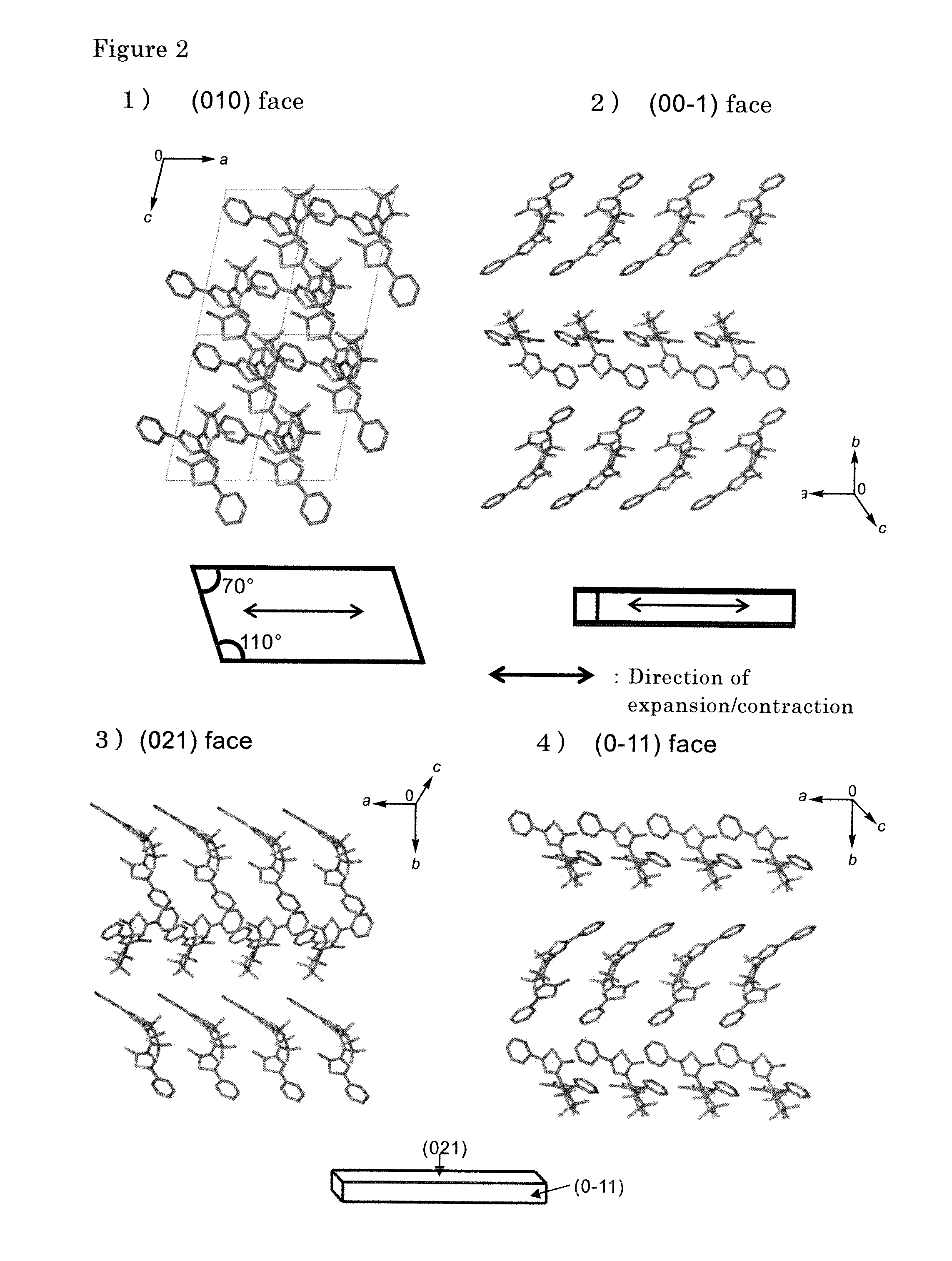
Disclosed is light-driven actuator element characterized in that, inter alia, it can be reduced to micrometer size, is rapidly responsive, and reversibly changes to enable repeated use. The light-driven actuator element includes a crystal of diarylethene compound which changes shape upon photoisomerization (e.g., the compound of Structural Formula (I) below, where R1 represents a hydrogen atom or methyl group and R2 represents a methyl group). The element can be a rod-shaped or plate-like microcrystal having a size on the order of micrometer. The element bends (or contracts) on irradiation with ultraviolet light and expands to return to the original size on irradiation with visible light.
Owner:IRIE
Optical recording material
InactiveUS20060097225A1Increased durabilityLiquid crystal compositionsPhotomechanical apparatusPhotoisomerizationSide chain
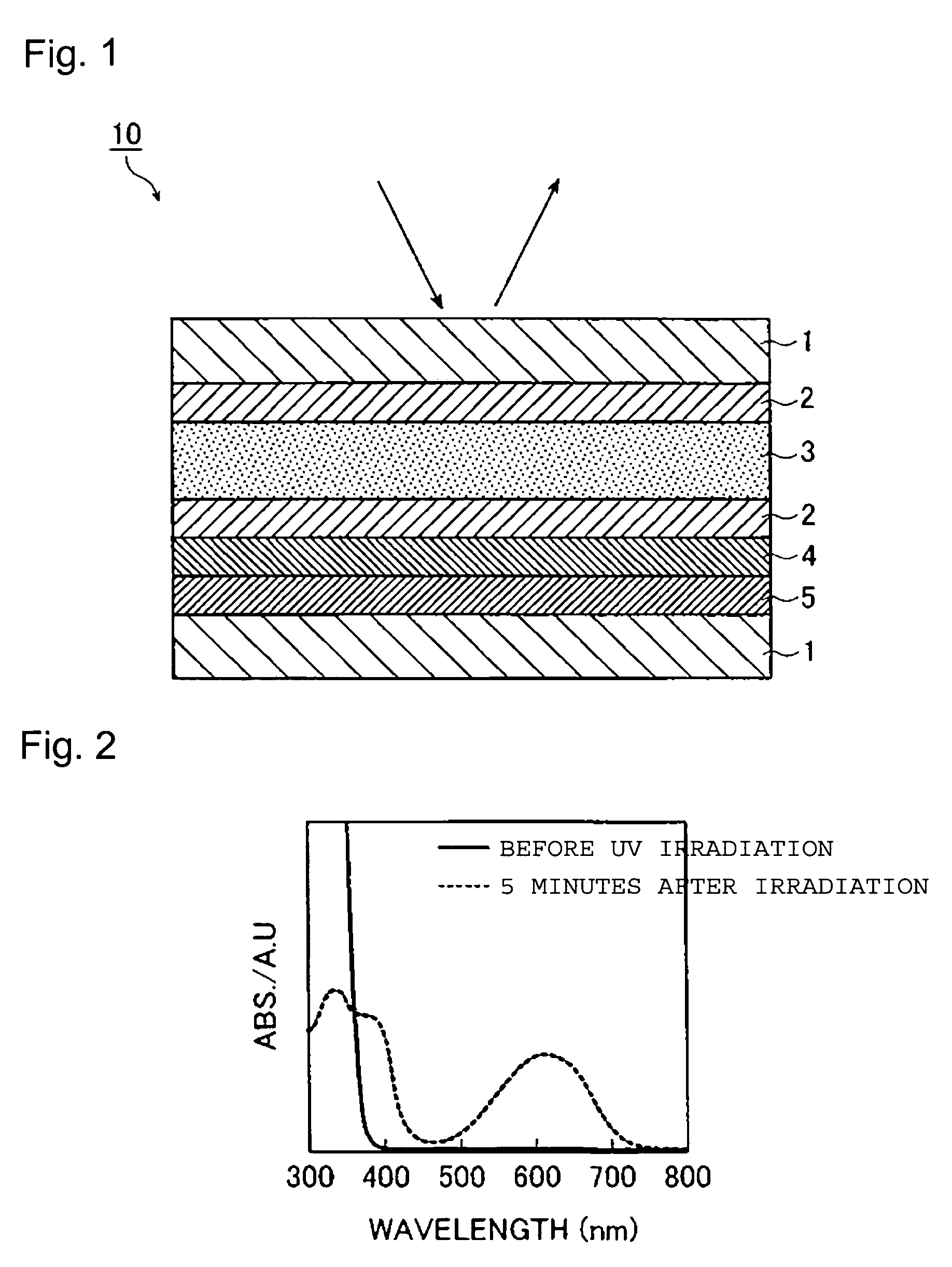
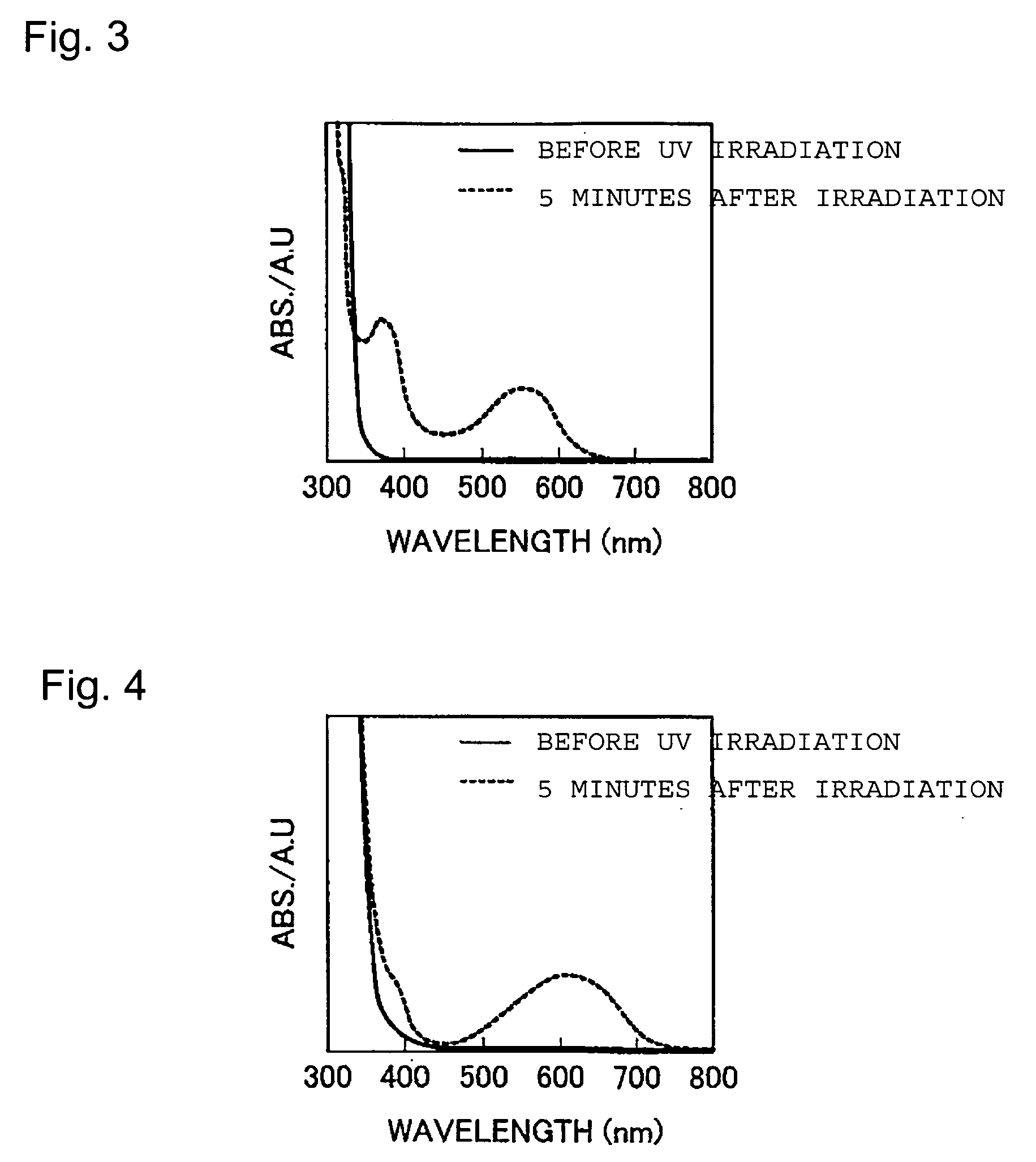
A novel photochromic liquid crystal comprising a diarylethene compound having both photochromic properties and liquid crystallinity. This compound is a photochromic diarylethene compound having a mesogen group in each of the two aryl groups. The photochromic liquid crystal provides a large change in birefringence by photoisomerization or phase transition, and has high durability against repeated changes in birefringence. A side-chain polymer liquid crystal containing the above photochromic liquid crystal is useful as an optical recording material. Such an optical recording material is capable of performing light modulation by irradiation with light near Tc thereof, and is applicable to photo operator devices, optical memories, and the like. Further, the optical recording material is useful as a material for a recording layer of an optical recording medium such as an optical disk or an optical memory card.
Owner:ASAHI GLASS CO LTD
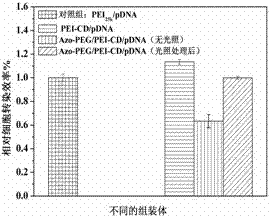
Preparation method of light-responsive pegized gene delivery system based on host-guest assembly
InactiveCN102268460AEasy to makeResponsiveGenetic material ingredientsOther foreign material introduction processesGene deliveryCyclodextrin
The invention discloses a preparation method of a light-responsive PEGylated gene delivery system based on host-guest assembly. Its steps are as follows: 1) Using N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid buffer solution, prepare polyethyleneimine grafted cyclodextrin solution with a concentration of 0.1-1 mg / mL and a concentration of 0.4-2 mg / mL azobenzene-modified polyethylene glycol solution; 2) Use the buffer solution in step 1) to prepare a DNA solution with a concentration of 50-500 μg / mL; 3) Mix the two solutions in step 1) and polymerize The molar ratio of cyclodextrin and azobenzene-modified polyethylene glycol contained in ethyleneimine-grafted cyclodextrin is 1:1-1:4, and the supramolecular compound solution is obtained after ultrasonication for 15 minutes and standing for 1 hour; 4) Add the solution prepared in step 3) to an equal volume of the solution in step 2), vortex to mix and let stand. The invention can improve its stability in physiological saline solution. Through the photoisomerization of azobenzene, the detachment of the PEG shell of the intracellular gene delivery system can be achieved, which can effectively improve the efficiency of gene transfection.
Owner:ZHEJIANG UNIV
Antibacterial composition for topical administration containing antibiotic
The present invention provides a safe antibacterial composition for topical administration which stably contains a penem antibiotic having a broad-spectrum and potent antibacterial activity while otherwise being chemically susceptible to hydrolysis, oxidation, photoisomerization or the like. The compositions of the present invention comprise an antibacterial composition for topical administration comprising a penem antibiotic or a pharmaceutically acceptable salt thereof in a non-aqueous base.
Owner:DAIICHI SUNTORY PHARMA CO LTD
Method for manufacturing liquid-crystal display
InactiveCN105431769AImprove display qualityThe preparation method is simple and easyNon-linear opticsPhotoisomerizationIn plane
This invention provides a method for manufacturing a liquid-crystal display that contains a photo-alignment film and can substantially improve display quality. Said method for manufacturing a liquid-crystal display containing a photo-alignment film has the following steps, in this order: a step (1) in which a film of a photo-alignment-film material is formed on a substrate, said photo-alignment-film material containing a solvent and a polymer that has an optical functional group that can undergo at least one chemical reaction selected from the group consisting of photodimerization, photoisomerization, and optical Fries rearrangement; a step (2) in which the aforementioned film is subjected to preliminary heating that vaporizes the aforementioned solvent; a step (3) in which the film, having been subjected to preliminary heating, is exposed to polarized light; and a step (4) that includes an operation in which the film, having been exposed to polarized light, is subjected to a primary heating process at a plurality of temperatures from low to high. This liquid-crystal display is a fringe-field-switching-mode liquid-crystal display or an in-plane-switching-mode liquid-crystal display with a pretilt angle that is effectively 0 DEG.
Owner:SHARP KK
Method for synthesizing 9beta,10alpha-dehydroprogesterone ketal through photochemical isomerization in micro-channel reactor
InactiveCN112275231AExtend the life cycleSuppress generationChemical/physical/physico-chemical microreactorsSteroidsPhotoisomerizationChemical reaction
The invention relates to a method for synthesizing 9beta,10alpha-dehydroprogesterone ketal through photochemical isomerization in a micro-channel reactor. The method comprises the following steps: dissolving a raw material, namely 9alpha,10beta-dehydroprogesterone ketal in a medium polar solvent to prepare a solution with the concentration of 10-50 g / ml, adding an antioxidant and organic alkali into the solution, and carrying out uniform stirring and mixing to prepare a photochemical reaction solution; and introducing nitrogen is into the obtained photochemical reaction solution for protection, and conducting illumination three times so as to realize photoisomerization of 9alpha,10 beta-dehydroprogesterone ketal. According to the invention, reaction time is greatly shortened, generation ofphotoisomerization reaction by-products is inhibited, conversion rate is high, and the method is suitable for industrial production.
Owner:JIANGSU ALPHA PHARM CO LTD
Quick-response reversible-photoisomerization perfluorinated-ether-chain azobenzene and preparation method therefor
InactiveCN104926683ASolve the problem of green environmental protectionNon-bioaccumulativeOrganic chemistryTenebresent compositionsStructural formulaAniline
The invention discloses quick-response reversible-photoisomerization perfluorinated-ether-chain azobenzene and a preparation method therefor. The perfluorinated-ether-chain azobenzene has a structural formula shown in the description. The preparation method comprises the steps: dissolving p-phenylene diamine and perfluoroether acyl fluoride, which serve as starting compounds, in an organic solvent so as to prepare (4-perfluoroether amide)phenylamine; diazotizing the compound by a hydrochloride / nitrite system, and then, enabling the diazotized compound to react with an alkali solution of phenol so as to prepare 4-perfluoroether amide-4'-hydroxyazobenzene; and in an anhydrous organic solvent, enabling 4-perfluoroether amide-4'-hydroxyazobenzene to react with methacryloyl chloride or acryloyl chloride so as to prepare 4-perfluoroether amide-4'-methacrylate azobenzene or 4-perfluoroether amide-4'-acrylate azobenzene. The compound disclosed by the invention has the advantages of reversible photoisomerization, quick response and high isomerization degree; a fluorinated ether chain is free of biological accumulation compared with a perfluoroalkyl hydrocarbon chain; and the compound contains an active functional group, i.e., propenyl which can participate in addition reaction or polymerization reaction, so that the field of application is broad.
Owner:DONGHUA UNIV
Photoactive polymers
InactiveCN1157425CLiquid crystal compositionsPhotomechanical apparatusPhotoisomerizationCrystallography
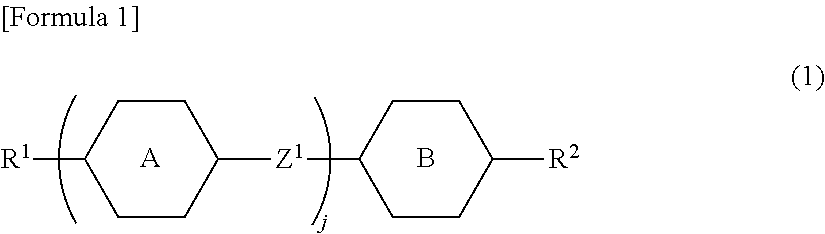
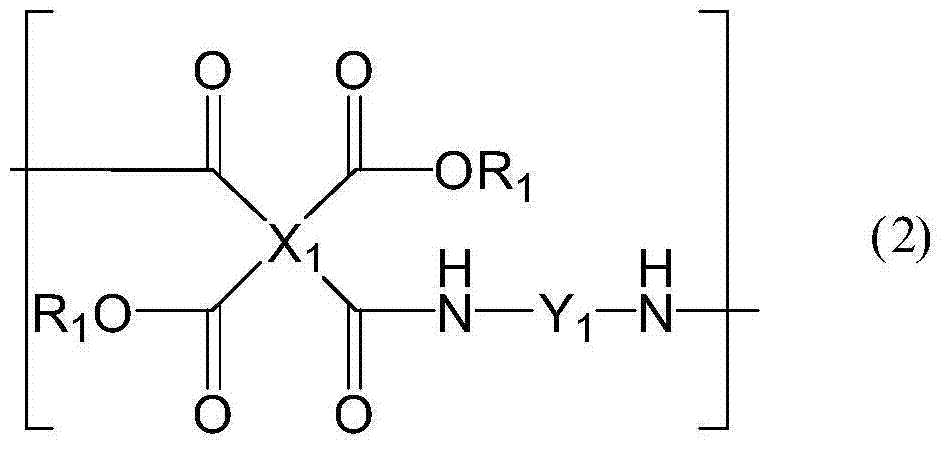
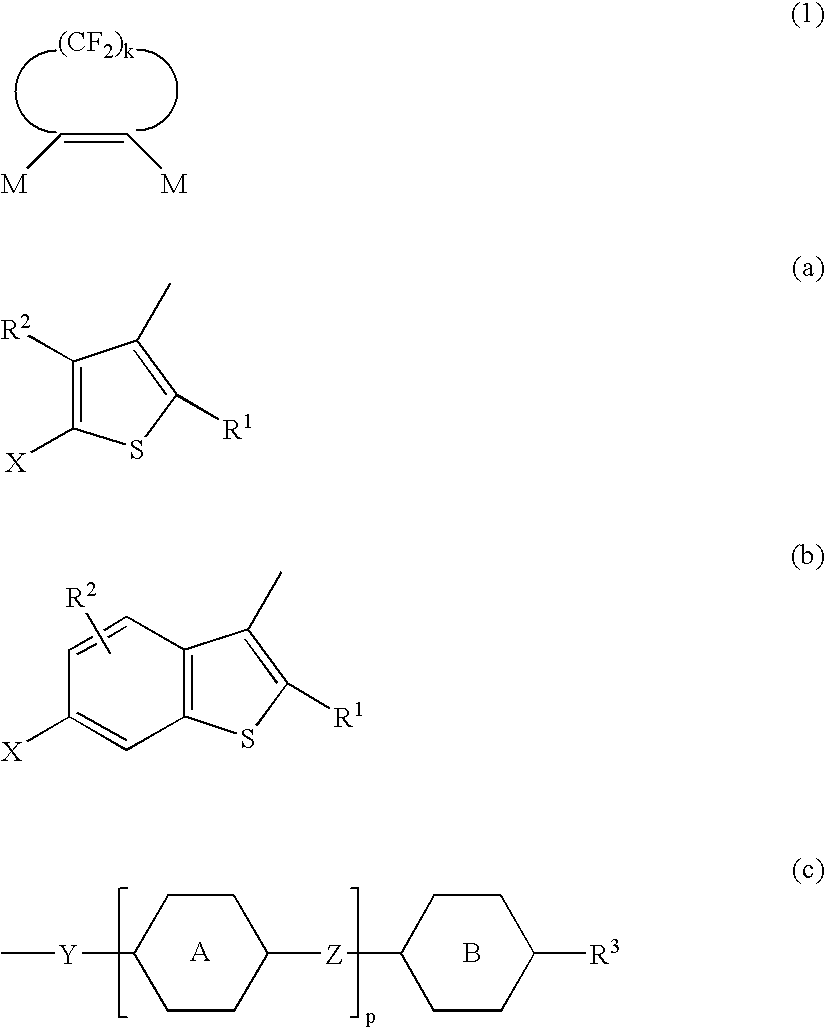
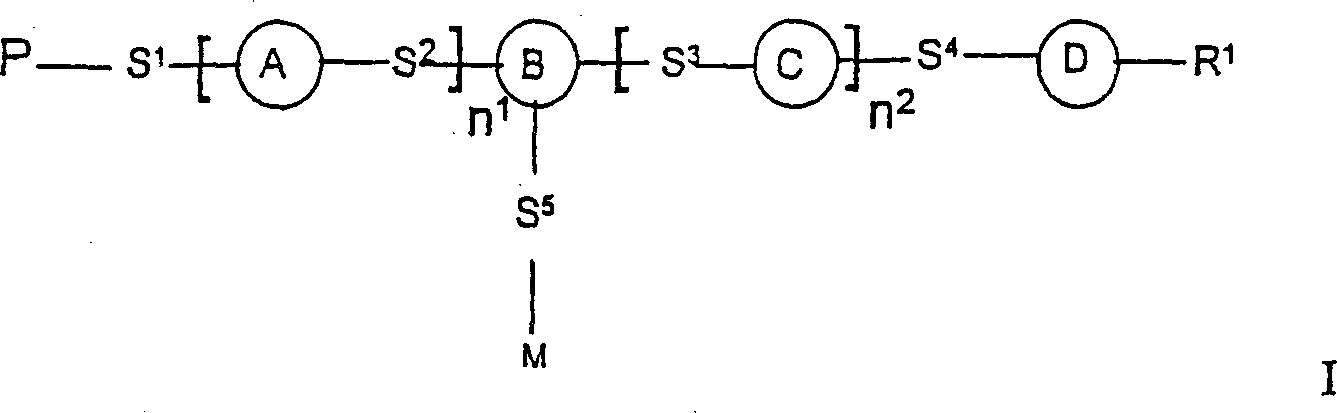
A photoactive polymer of the general formula I:in which:P is a photoactive group which can photoisomerise and / or photodimerise;B represents an aromatic or alicyclic group, or B further represents a nitrogen atom or -CR<2>-;A, C, D each independently of the other represents an aromatic or alicyclic group;M represents a repeating monomer unit in a homo- or copolymer;S<1>, S<2>, S<3>, S<4>, S<5 >represent a single covalent bond or a spacer unit;n<1>, n<2 >are each independently a positive integer up to 2 with the proviso that n<1>+n<2><=2;R<1 >is a hydrogen atom, or a straight-chain or branched alkyl residue wherein R<2 >represents a hydrogen atom or lower alkyl.The photoactive polymers may be used as orientation layers for liquid crystals and in the construction of unstructured and structured optical elements and multi-layer systems.
Owner:ROLIC AG
Method for manufacturing liquid crystal display device
ActiveUS20160178968A1Improve display qualityAvoid failureTube/lamp screens manufactureCoatingsPhotoisomerizationIn plane
The present invention provides a method for manufacturing a liquid crystal display device including a photo-alignment film and capable of sufficiently improving the display quality. The method for manufacturing a liquid crystal display device of the present invention is a method for manufacturing a liquid crystal display device including a photo-alignment film. The method for manufacturing a liquid crystal display device successively includes a step (1) of forming on a substrate a film from a photo-alignment-film material that contains a solvent, a polymer including a photo-functional group that is capable of causing at least one chemical reaction selected from the group consisting of photodimerization, photoisomerization, and photo-Fries rearrangement, and a polymer including a polyamic acid backbone and free from the photo-functional group; a step (2) of pre-heating the film to evaporate the solvent; a step (3) of irradiating the pre-heated film with polarized light; and a step (4) of main-heating the polarized-light-irradiated film. The liquid crystal display device is of an in-plane switching mode or a fringe field switching mode in each of which a pre-tilt angle is substantially 0°.
Owner:SHARP KK
Rapid-response photoisomerization 4-perfluoroalkyl azobenzene allyl ether and preparation method thereof
The invention discloses rapid-response photoisomerization 4-perfluoroalkyl azobenzene allyl ether and a preparation method thereof. The general structural formula of 4-perfluoroalkyl azobenzene allyl ether is shown in the Specification. The preparation method includes the following steps: dissolving the starting compounds, 4-iodoaniline and fluorine-containing alkyl iodide, into a high boiling point solvent, and under the protection of nitrogen, heating, and catalyzing the solvent by copper powder to obtain fluoroalkyl aniline; after diazotizing fluoroalkyl aniline using hydrochloric acid / nitrite system at a temperature of 0-5 DEG C, enabling the diazotized solution to react with the phenol alkaline solution to obtain perfluoroalkyl azo phenol; under the protection of nitrogen, heating perfluoroalkyl azo phenol and 3-bromopropylene in an anhydrous and oxygen-free polar solvent under a weak base condition to obtain 4-fluoroalkyl azobenzene propenyl ether. The fluorine-containing azobenzene compound photoisomerization provided by the invention can response rapidly, has an active functional group propenyl, is capable of participating in addition reactions or polymerization reactions, and can be applied widely.
Owner:DONGHUA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com